
Carbon12 Electron configuration, Carbon element, Atom
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. Electron configuration of Carbon (C) [He] 2s 2 2p 2: 1s 2 2s 2 2p 2: 2, 4: 7: Electron configuration of Nitrogen (N) [He] 2s 2 2p 3: 1s 2 2s 2 2p 3: 2, 5: 8: Electron configuration of.
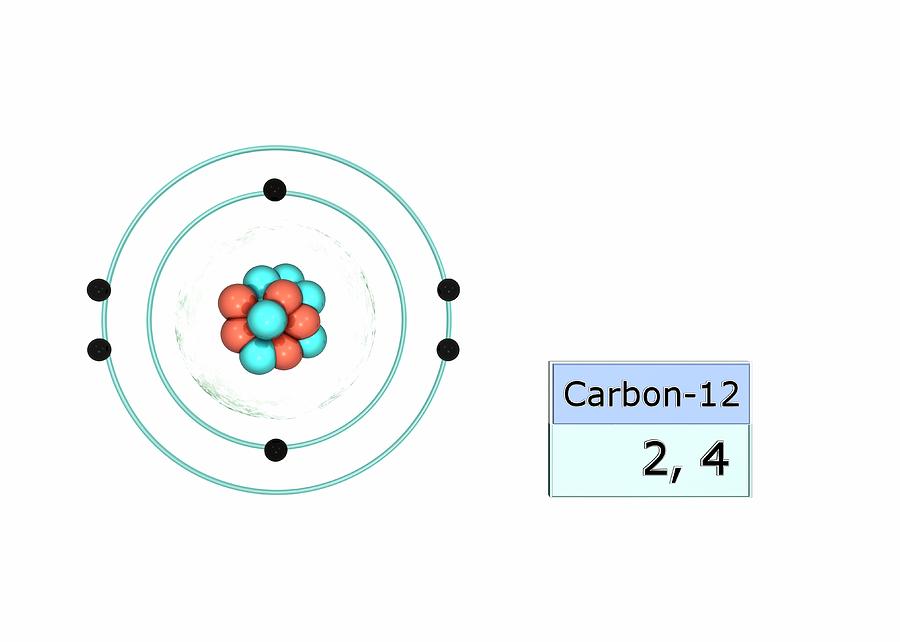
Carbon Electron Configuration Photograph by Photo Libary Pixels
For hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (Figure 6.29"), and the electron configuration is written as 1 s1 and read as "one-s-one.". A neutral helium atom, with an atomic number of 2 ( Z = 2), has two electrons. We place one electron in the orbital that is lowest in.

Electronic Configuration for Carbon spdf Trick Chemistry Atomic Number 6 YouTube
It is composed of one electron from each atom and is thus a two-electron bond. Each atom contributes with one hybridized orbital, for example the sp ones in ethane C2H6. Other kinds of hybridizations can also be used to create single bonds, for instance sp: there is no hybridization requirement to bond two carbon atoms (figure ).

Carbon Element With Reaction, Properties, Uses, & Price Periodic Table
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write Electron Configurations. Step-by-step tutorial for writing the Electron Configurations.

Carbon(C) electron configuration and orbital diagram
Electronic Structure of Atoms and Molecules Electronic Configurations
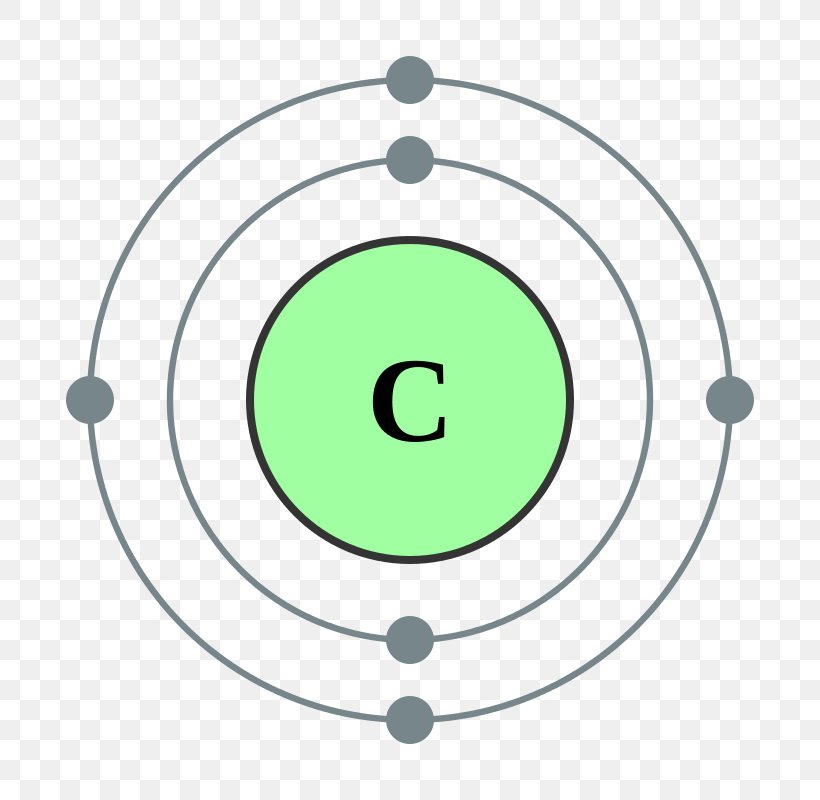
Electron Configuration Electron Shell Valence Electron Carbon, PNG, 800x800px, Electron
What are electron configurations? The cells in our bodies are masters of quantum physics---they've figured out the complicated dance of atoms and electrons, and they use this knowledge to build an endlessly complex series of signalling pathways and genetic circuits.
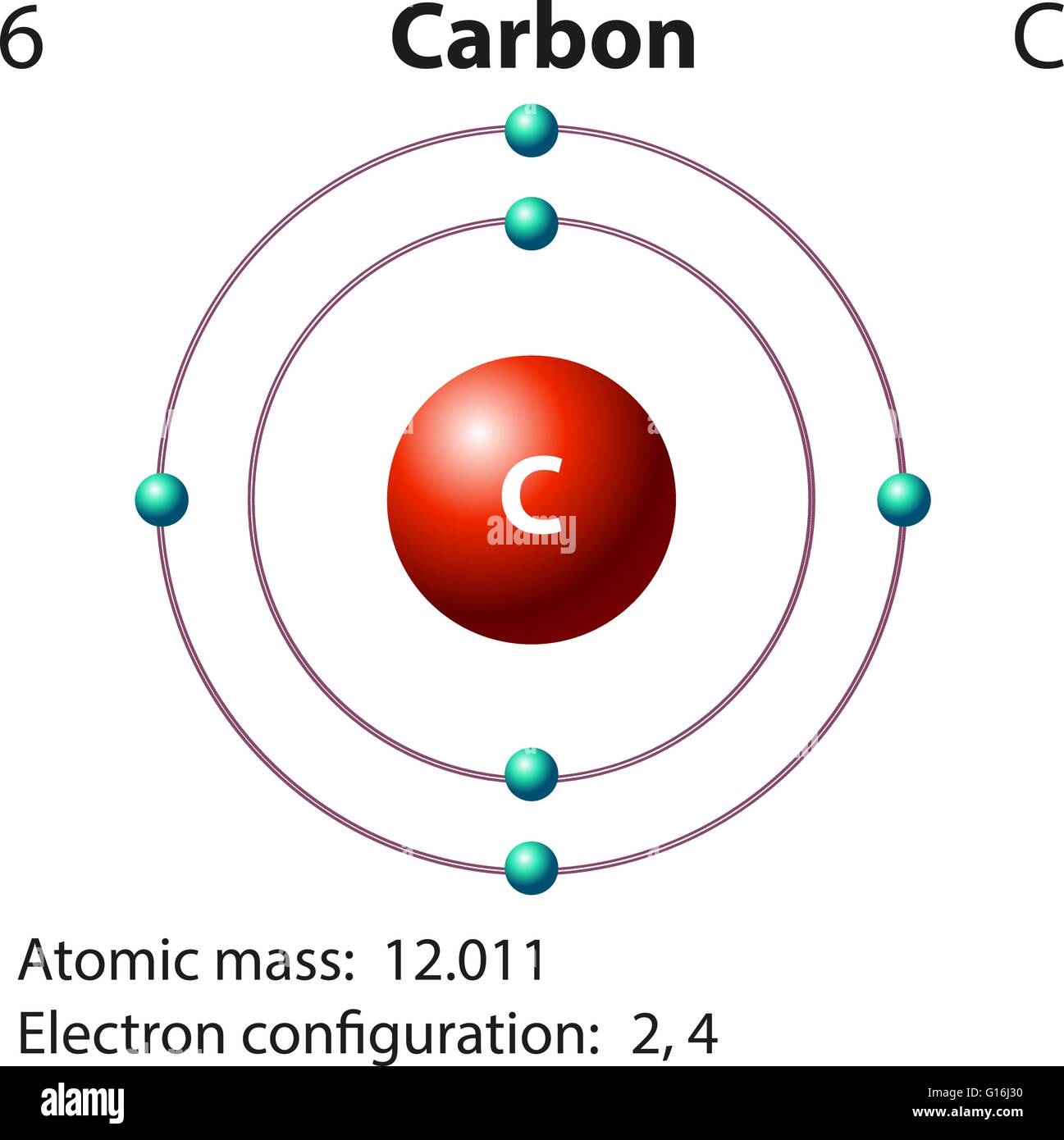
Carbon atom diagram hires stock photography and images Alamy
An atom's ground state electron configuration describes how the electrons have distributed among the orbital shells and subshells. According to the electron configuration chart , electrons in an atom occupy orbitals according to their increasing energy, with each orbital having a maximum of two paired electrons with opposite spins .

Electronic Configuration Explanation & Examples Embibe
A step-by-step description of how to write the electron configuration for Carbon (C). In order to write the C electron configuration we first need to know t.

Carbon Atom Science Notes and Projects
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,

Carbon Electron Configuration YouTube
Carbon is a chemical element with atomic number 6 which means there are 6 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.

3d render of atom structure of carbon isolated over white background Stock Photo Alamy
Electronic configuration of the Carbon atom. Valence electrons. Orbital diagram. Carbon electron configuration. ← Electronic configurations of elements . C (Carbon) is an element with position number 6 in the periodic table. Located in the II period. Melting point: 3550 ℃.

Carbon, atomic structure Stock Image C018/3687 Science Photo Library
Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.
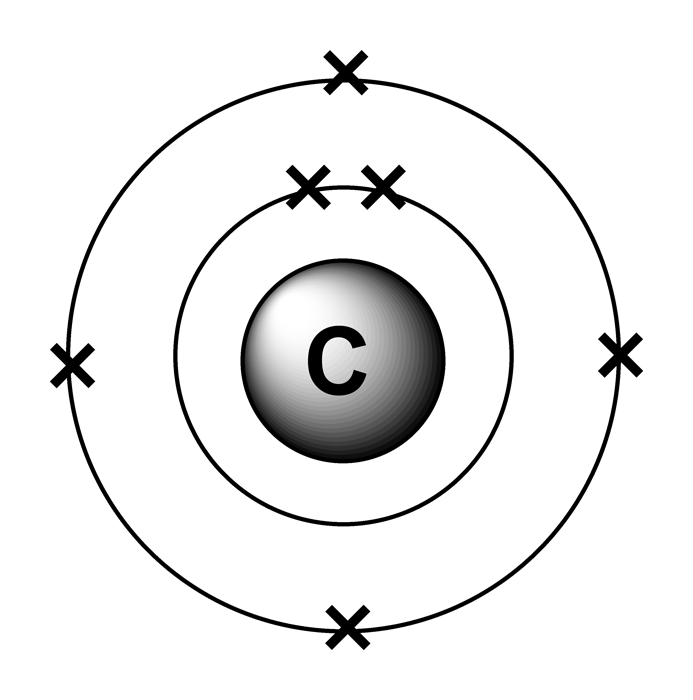
Electron arrangements
About Transcript Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan. Questions Tips & Thanks Want to join the conversation? Sort by:
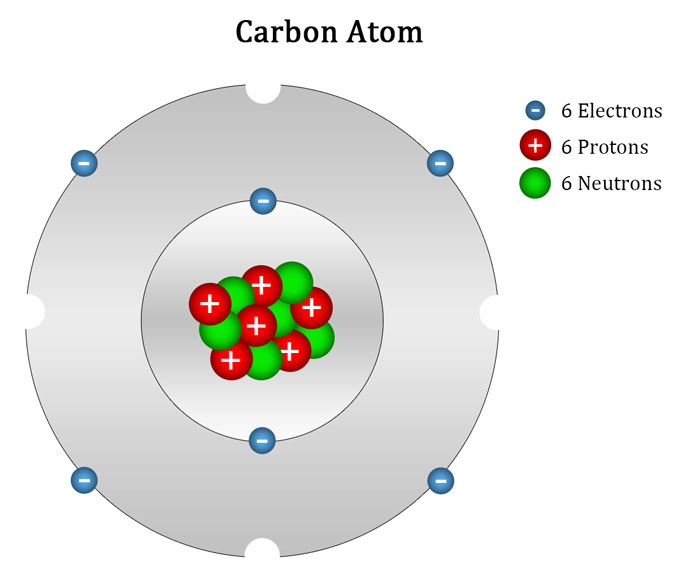
Carbon Atom Ascension Glossary
The number of protons in an atom. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point. The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the.

Orbital Diagram For Carbon (C) Carbon Electron Configuration
Electron configurations The ground-state electronic configurations of atoms of these carbon group elements show that each has four electrons in its outermost shells. As has been explained, if n represents the outermost shell ( n being two for carbon, three for silicon, etc.), then these four electrons are represented by the symbols ns2np2.

Electron Distribution Diagram Of Carbon
A Carbon atom is a neutral atom that has 6 atomic numbers which imply it has a total of 6 electrons. As per the Aufbau rule, the electrons will be filled into 1s orbital first then 2s, then 2p…so on. Now, for the electron configuration of Carbon, the first 2 electrons will go in 1s orbital since s subshell can hold a maximum of 2 electrons.