
Bohr Rutherford Diagram For The First 20 Elements
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.

PPT Bohr Rutherford Atomic Model PowerPoint Presentation, free
Because the Bohr Model is a modification of the earlier Rutherford Model, some people call Bohr's Model the Rutherford-Bohr Model. The modern model of the atom is based on quantum mechanics. The Bohr Model contains some errors, but it is important because it describes most of the accepted features of atomic theory without all of the high-level.

BohrRutherford Diagrams YouTube
This Bohr-Rutherford model explains the structure of the atom, placement of different atomic species inside the atom as well as the charge on different atomic particles. It also explained why electrons remain confined to their shells instead of falling inside the nucleus.
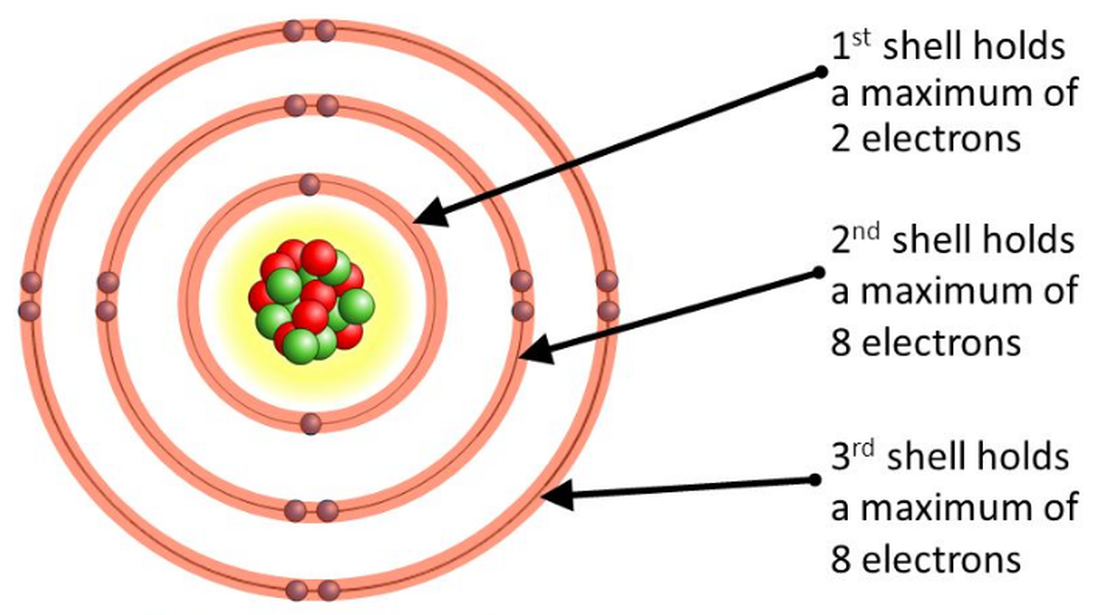
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons
Silicon has 2 electrons in its first shell, 8 in its second, 4 in its third.Check me out: http://www.chemistnate.com
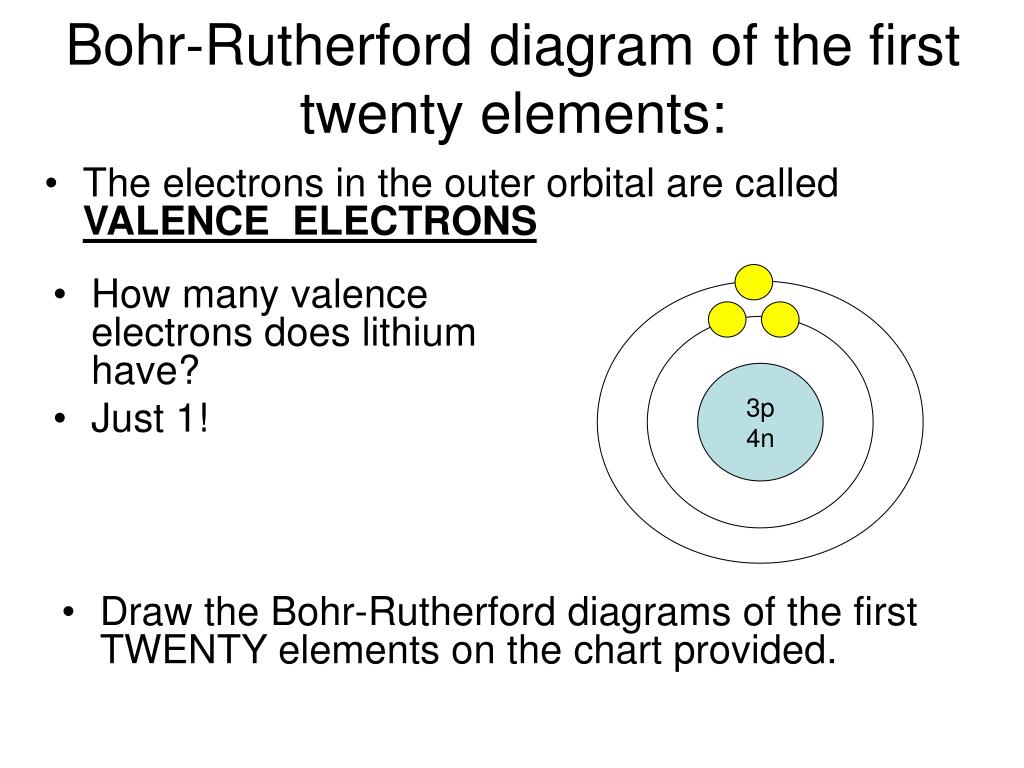
PPT BohrRutherford Diagrams for Atoms PowerPoint Presentation, free
0:00 / 5:40 Bohr-Rutherford Diagram of NaCl (sodium chloride, table salt) chemistNATE 260K subscribers Subscribe Subscribed Share 6.7K views 3 years ago NaCl, sodium chloride, is an IONIC.
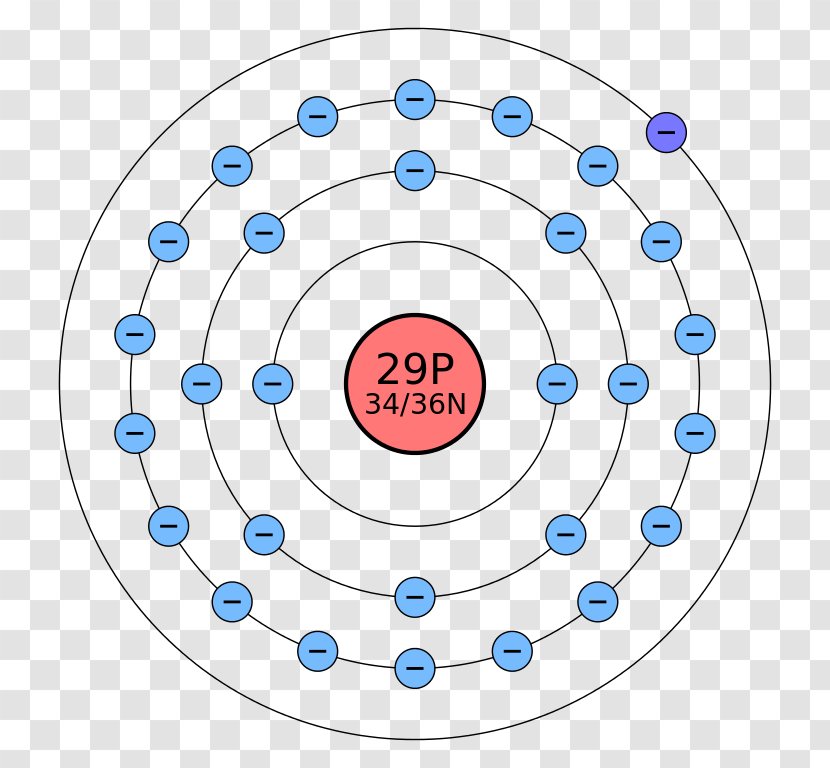
Bohr Model Atom Electron Shell Copper Rutherford Elements Vector
A Bohr Rutherford diagram, also known as a Bohr model or Rutherford model, is a visual representation of an atom's electron configuration. It shows the arrangement of electrons in the various energy levels or shells surrounding the nucleus of an atom. To create a Bohr Rutherford diagram, follow these steps:

Bohr Rutherford Diagram For First 20 Elements Diagram For You
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
_0.jpg?itok=RuBFwyf1)
Le modèle atomique de RutherfordBohr Alloprof
Non-Metals. Non-metals - Tend to have 4, 5, 6, or 7 electrons in their outer orbits (shells). They gain electrons to form negative ions (anions) They gain electrons, thus they have the same electron arrangement as the Noble gas in the same row. Try to make a Bohr-Rutherford ion for phosphorous. 15 31 P.

PPT Bohr Rutherford Atomic Model PowerPoint Presentation, free
How to Draw the Bohr-Rutherford Diagram for Calcium chemistNATE 260K subscribers Subscribe Subscribed 990 92K views 4 years ago Calcium has 2 electrons in its first shell, 8 in its second, 8 in.
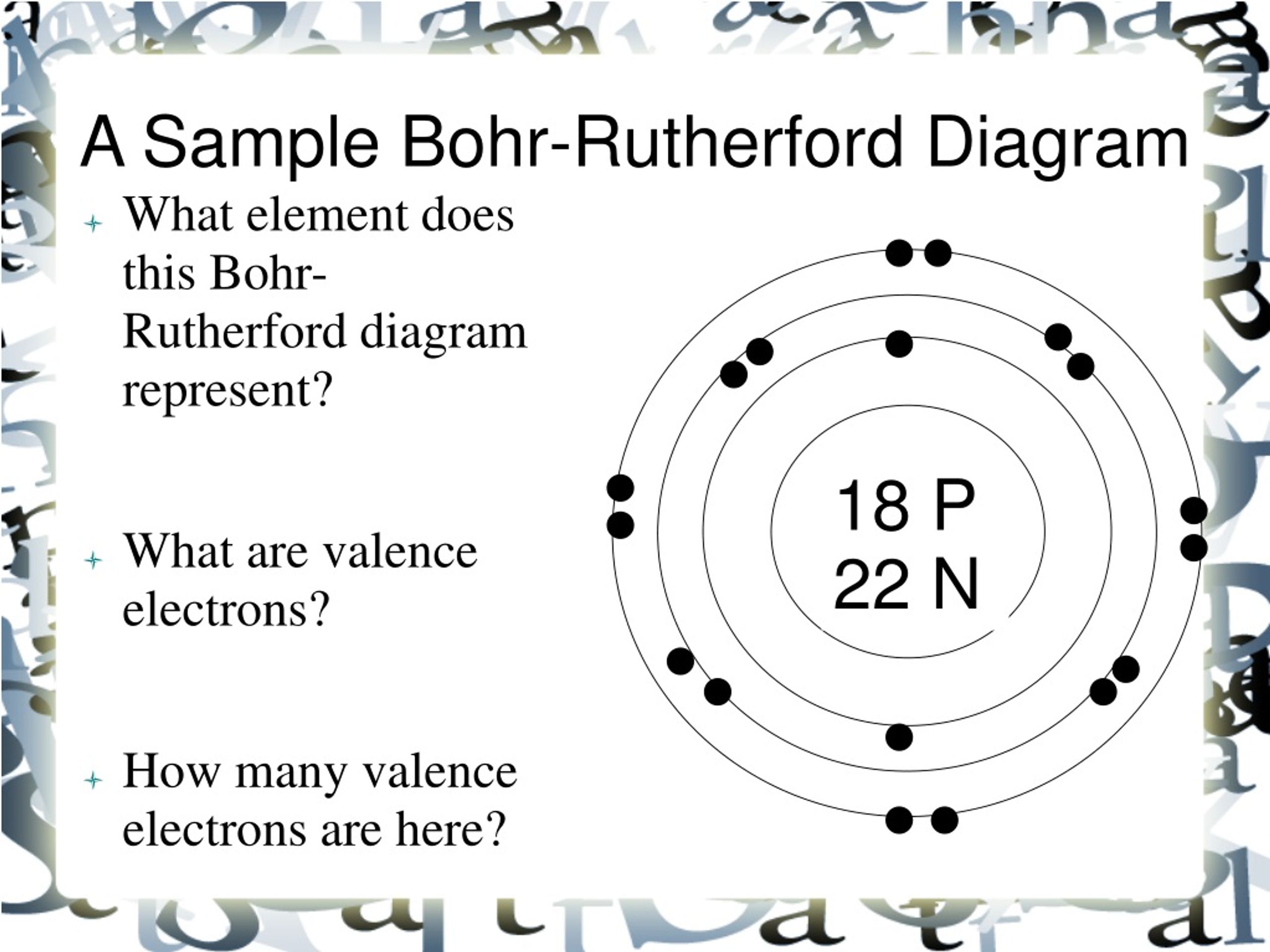
PPT BohrRutherford Diagrams PowerPoint Presentation, free download
How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on.

bohr rutherford diagram
The Bohr-Rutherford diagram contains a circle at the center representing the nucleus of the atom. The circle contains the chemical symbol, number of protons, number of neutrons present in the atom. The circular arcs are drawn around the circle representing the shells of the atoms for the electrons. As per the shell capacity of holding the.
[Solved] Create the BohrRutherford diagram for the following elements
Rutherford atomic model, nuclear atom, or planetary model of the atom Key People: Ernest Rutherford atom Top Questions What was the impact of Ernest Rutherford's theory? Rutherford model, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford.
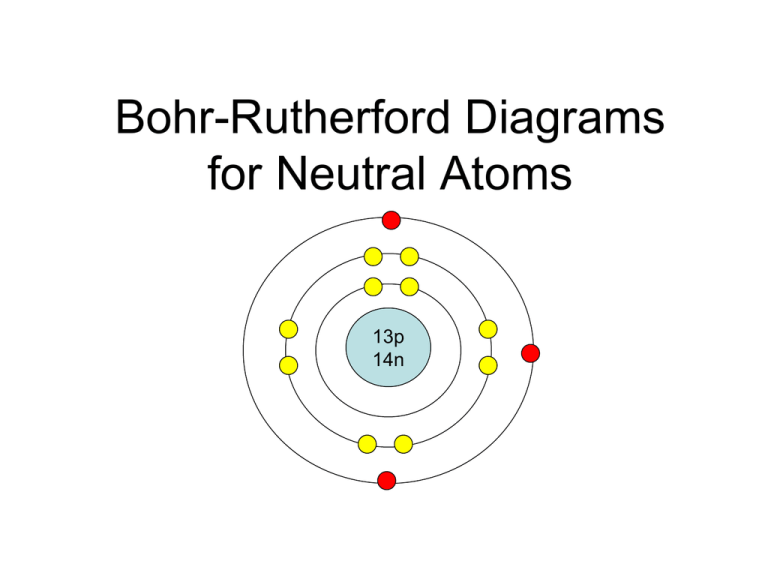
BohrRutherford diagrams for atoms
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV

Bohr's Atomic Model — Overview & Importance Expii
This page contains materials for the session on the atomic models of Rutherford and Bohr. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.
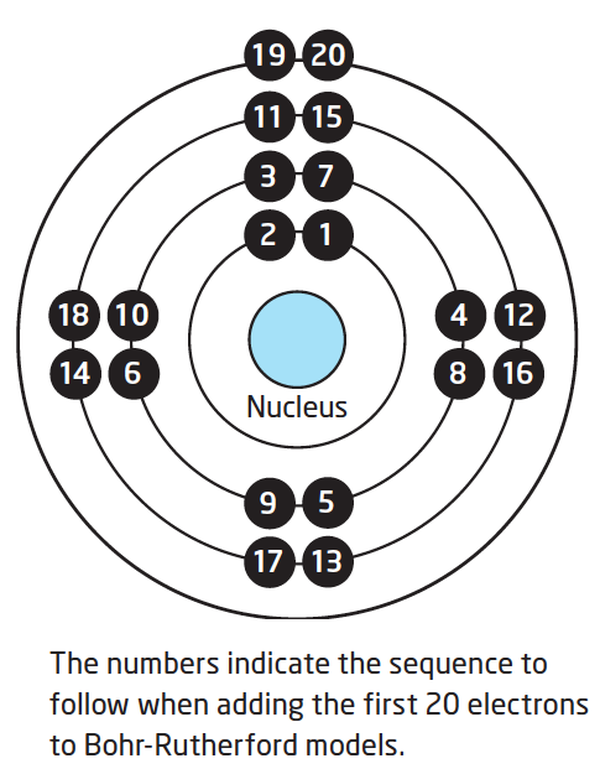
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons
More. Embed this widget ». Added Aug 1, 2010 by JB1295 in Chemistry. Gives the Lewis Dot structure for any element. Send feedback | Visit Wolfram|Alpha. Lewis Dot Diagram of. Submit. Get the free "Bohr Model Widget" widget for your website, blog, Wordpress, Blogger, or iGoogle.
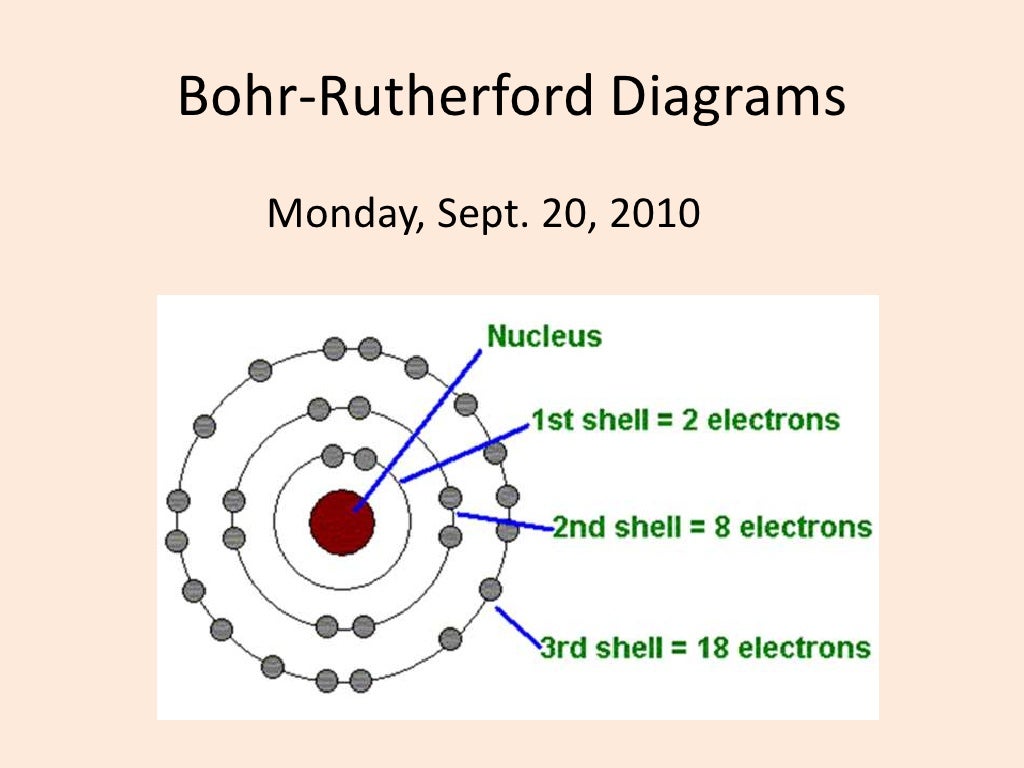
Bohr rutherford diagrams
How to draw a Bohr-Rutherford Diagram? Draw a nucleus -write the number of protons and neutrons inside the nucleus. Draw orbitals around the nucleus. Represent electrons as pairs of dots in the orbitals. Draw electrons as dots on the rings that represent the energy levels. Each ring has a maximum number of electrons that it can hold.