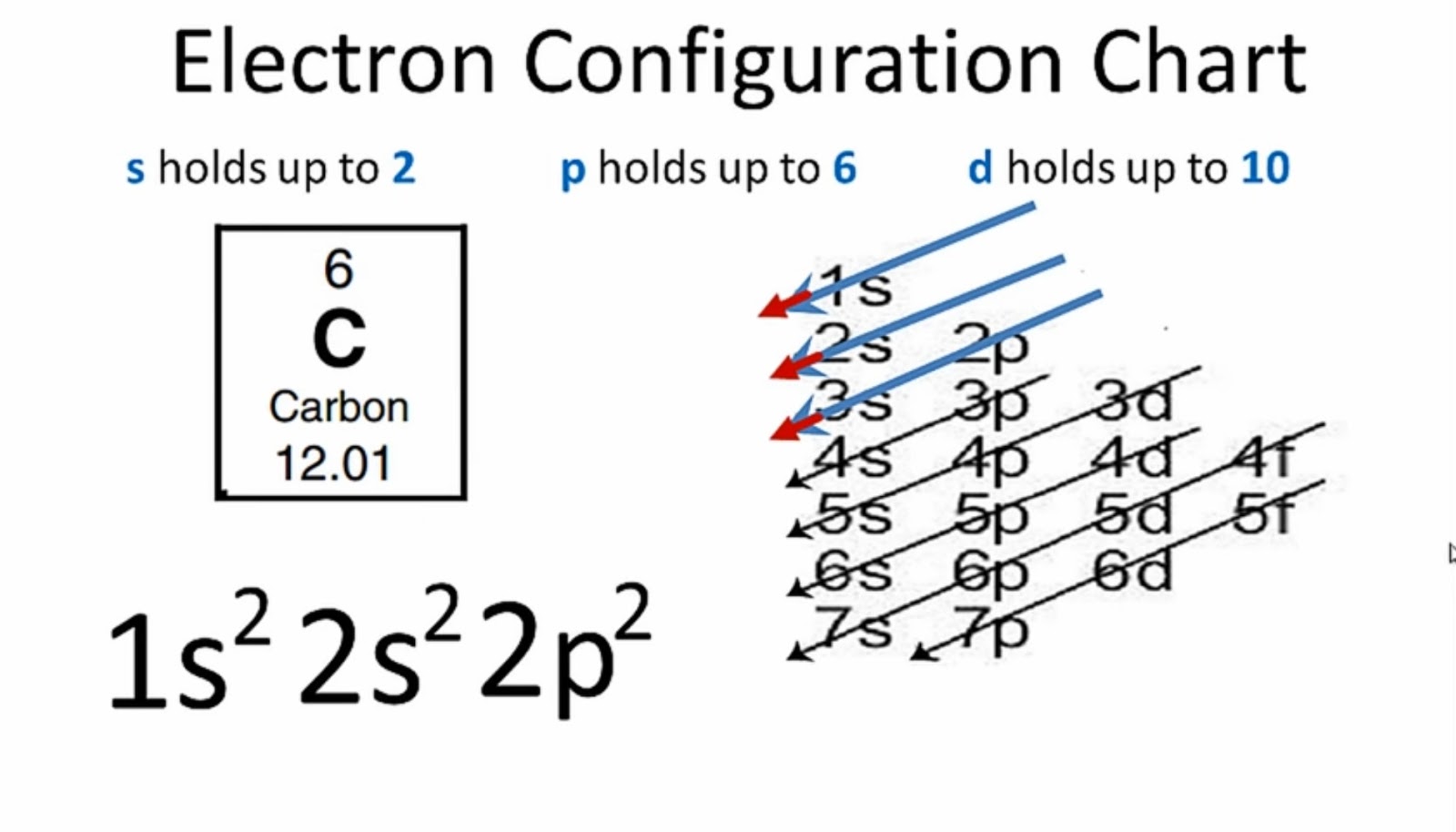
What Is the Carbon(C) Electron Configuration?
To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of.
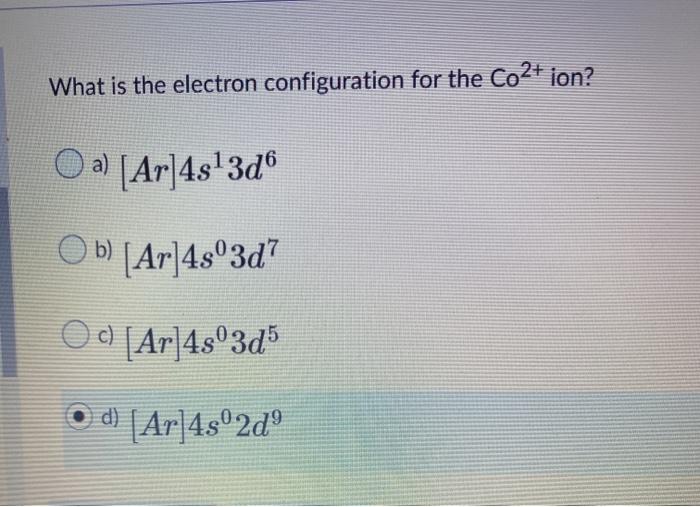
Solved What is the electron configuration for the Co2+ ion?
The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the elements up through number 104.
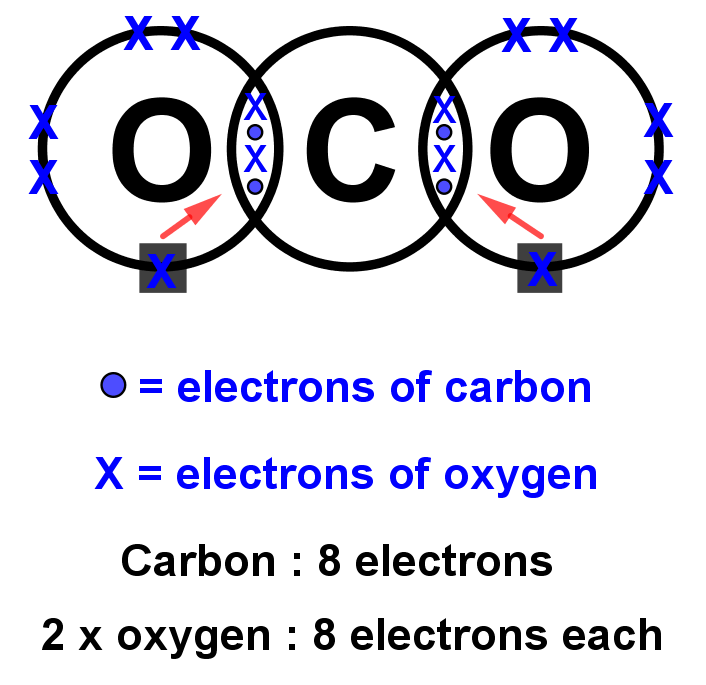
13+ Co2 Dot And Cross Diagram Robhosking Diagram
The electron configuration of Co is 1s2 2s2 2p6 3s2 3p6 3d7. Co requires three more valence electrons in its d-orbital to attain full filled configuration. What is the complete electron configuration of Co 2+? The complete electron configuration of Co2+ is 1s2 2s 2 2p6 3s2 3p6 3d7.Co2+ have 7 valence electrons in the subshell.

Silver Electron Configuration
The n + l n + l rule tells you the order in which atomic orbitals are filled, and according to the rule the 4s 4 s orbital is occupied before the 3d 3 d orbital because it has lower energy. Thus, the electron configuration of Mn M n is [Ar]3d54s2 [ A r] 3 d 5 4 s 2 while that of Co C o is [Ar]3d74s2 [ A r] 3 d 7 4 s 2 .
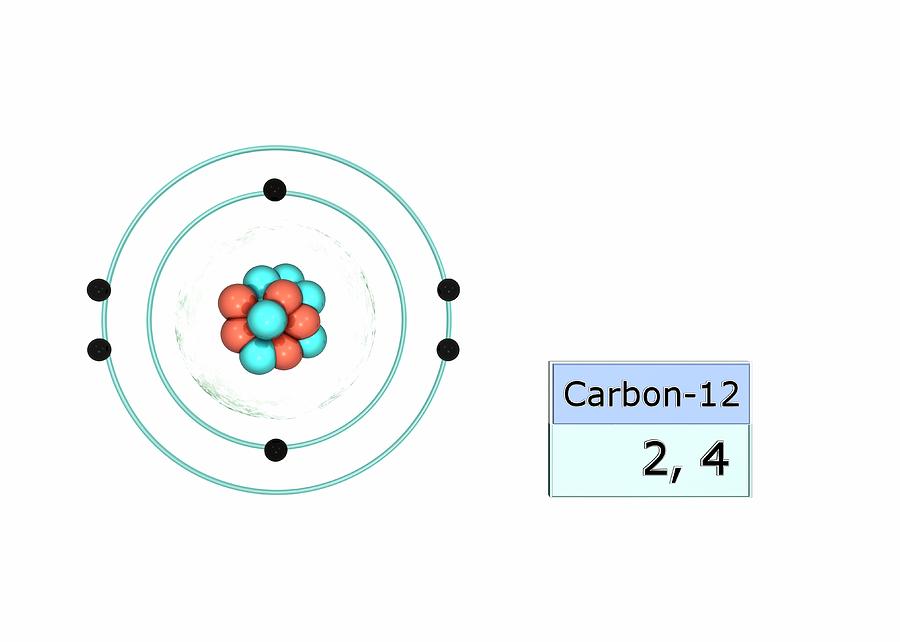
Carbon Electron Configuration Photograph by Photo
Here, the electron configuration of cobalt ion(Co 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7. This cobalt ion(Co 2+) has twenty-seven protons, thirty-two neutrons, and twenty-five electrons. Also, cobalt has one more ion. That is Co 3+. Co - 3e - → Co 3+ Here, the electron configuration of cobalt ion(Co 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6.
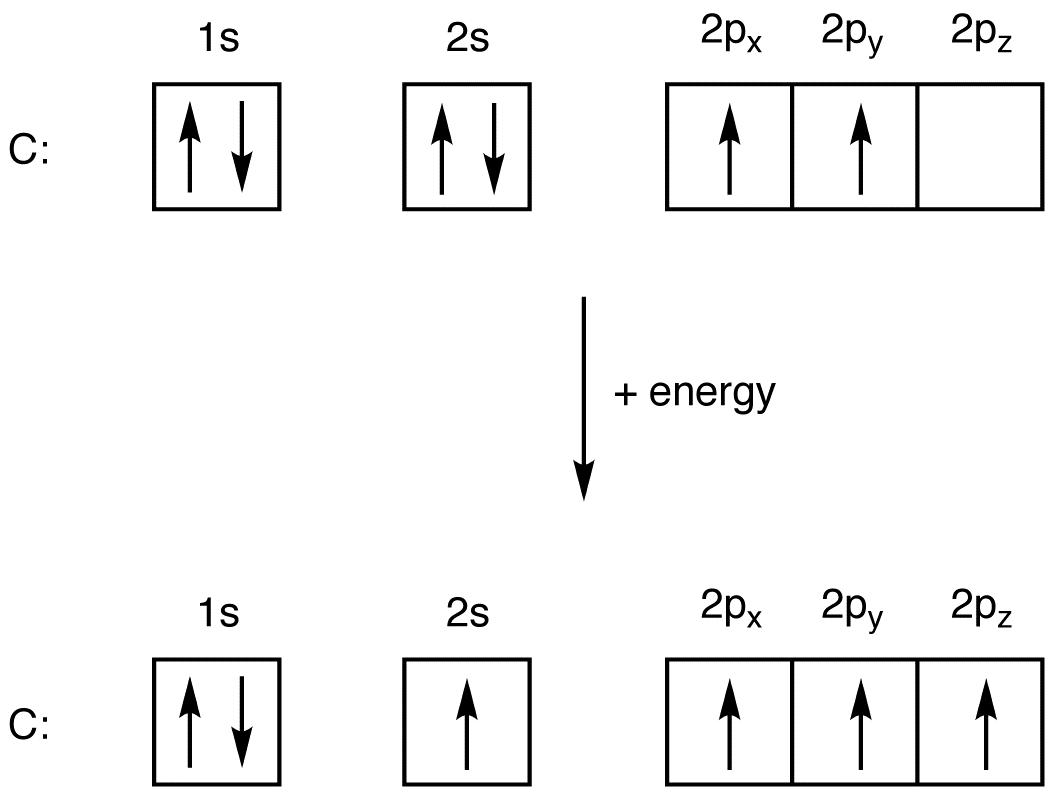
Electron Configuration Of Carbon
To determine the number of valence electrons for CO2, the Carbon dioxide molecule, we'll use the Periodic Table. Organizing the Periodic Table by Group, ski.

So far, we’ve used 16 of the CO2 Lewis structure’s total 16 outermost
The Octet Rule The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. This allows each halogen atom to have a noble gas electron configuration.

Carbon Electron Configuration
The electron configuration of Cobalt is [Ar]4s 2 3d 7. When observing Cobalt 3+, we know that Cobalt must lose three electrons. The first two to go are from the 4s orbital and Cobalt becomes:[Ar]4s 0 3d 7. Then, the next electron leaves the 3d orbital and the configuration becomes: [Ar]4s 0 3d 6. Thus, we can see that there are six electrons.
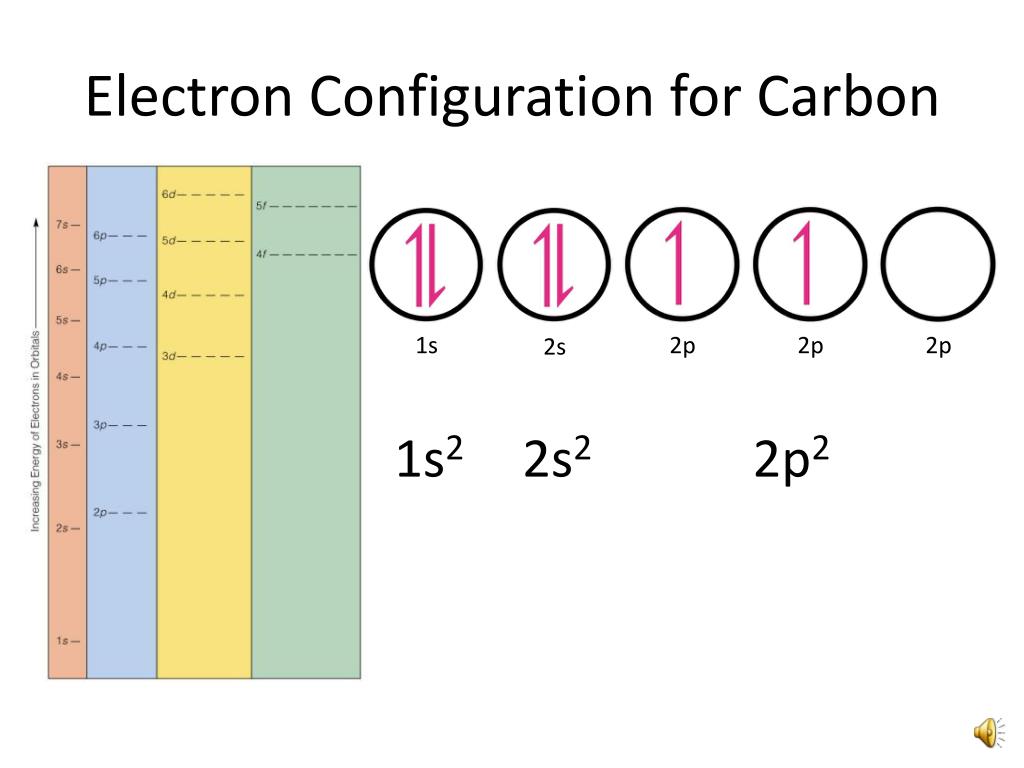
PPT Orbital Filling Electron Configurations PowerPoint Presentation
Losing the two 4s electrons leaves a positive charge of +2 and seven 3d electrons. Answer link. Co^ (2+) would most likely be 1s^2 2s^2 2p^6 3s^2 3p^6 4s^0 3d^7, and Co would have 4s^2 instead (the rest otherwise the same). The two outermost electrons are the 4s electrons so these are the two electrons that most likely will be lost.

Electron Geometry for CO2 (Carbon Dioxide) YouTube
1. Introduction Electron configuration describes the distribution of electrons within an atom. The concept of electron configuration has been introduced since the discovery of Bohr atomic model. However, the electron configuration referred in this study was one that derived from the later atomic model - the quantum mechanics atomic model.
26 Draw The Orbital Diagram For The Ion Co2+. Wiring Database 2020
An electrochemical conversion of carbon dioxide into chemical fuels is a promising approach to store the renewable energy sources 2. However, a critical challenge toward efficient CO 2 reduction.
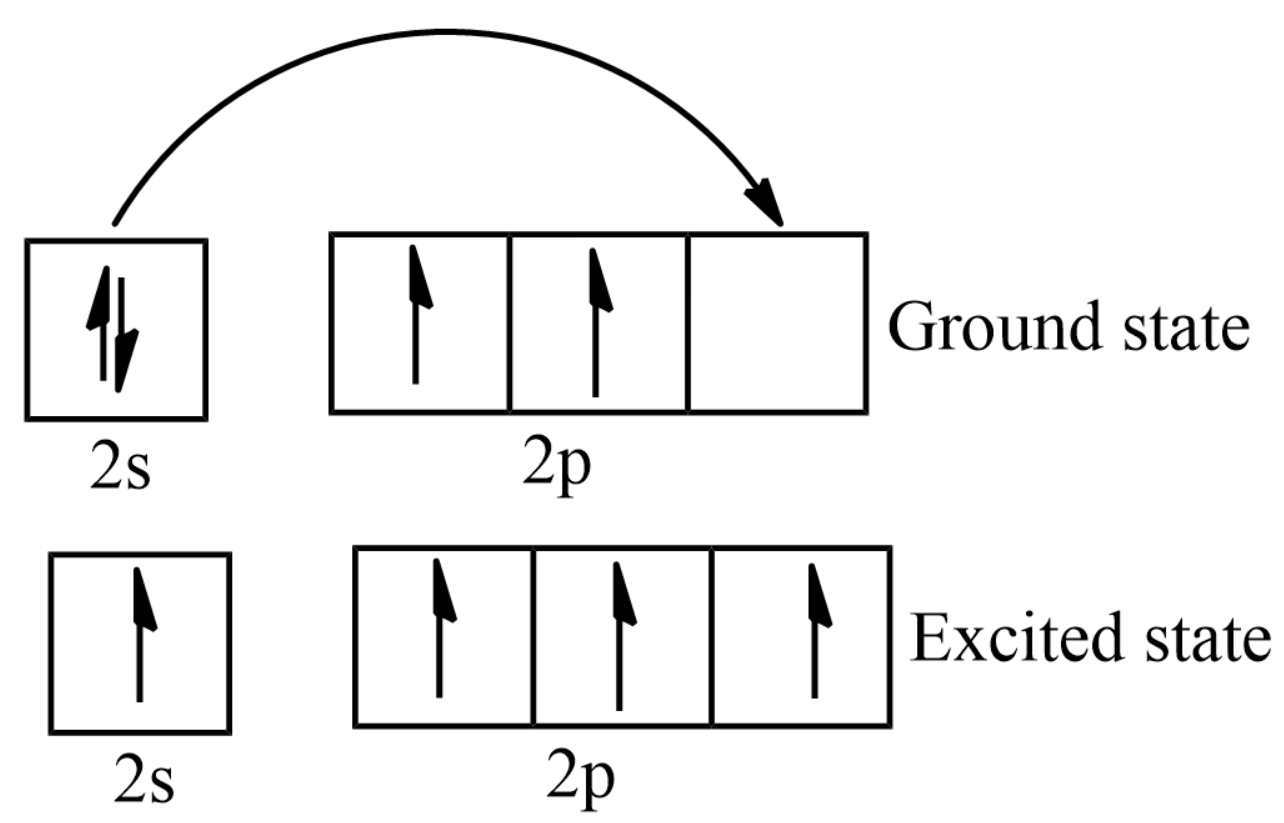
What is the excited state of carbon?
To write electron configuration of an element, locate its symbol in ADOMAH Periodic Table and cross out all elements that have higher atomic numbers. For example, if you need to write electron configuration of Erbium (68), cross out elements 69 through 120. Notice numbers 1 through 8 at the base of the table.

Electronic Configuration for Carbon spdf Trick Chemistry Atomic
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).

Electron Configuration for Co, Co2+, and Co3+ (Cobalt and Cobalt Ions
Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.

Orbital Diagram For Cobalt
PROBLEM 3.1.13 3.1. 13. Thallium was used as a poison in the Agatha Christie mystery story "The Pale Horse.". Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium. Answer.

Carbon Element With Reaction, Properties, Uses, & Price Periodic Table
Hello Guys,Determining the electron configuration of any element is an easy and quick process, given that you know all the required information and general c.