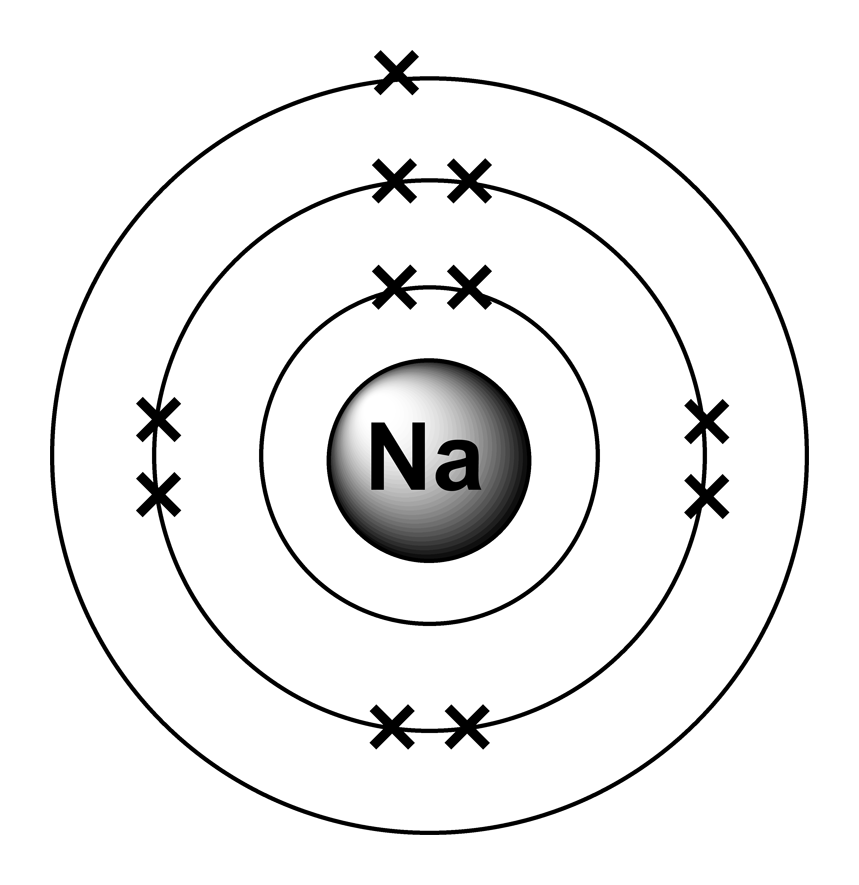
Electron arrangements
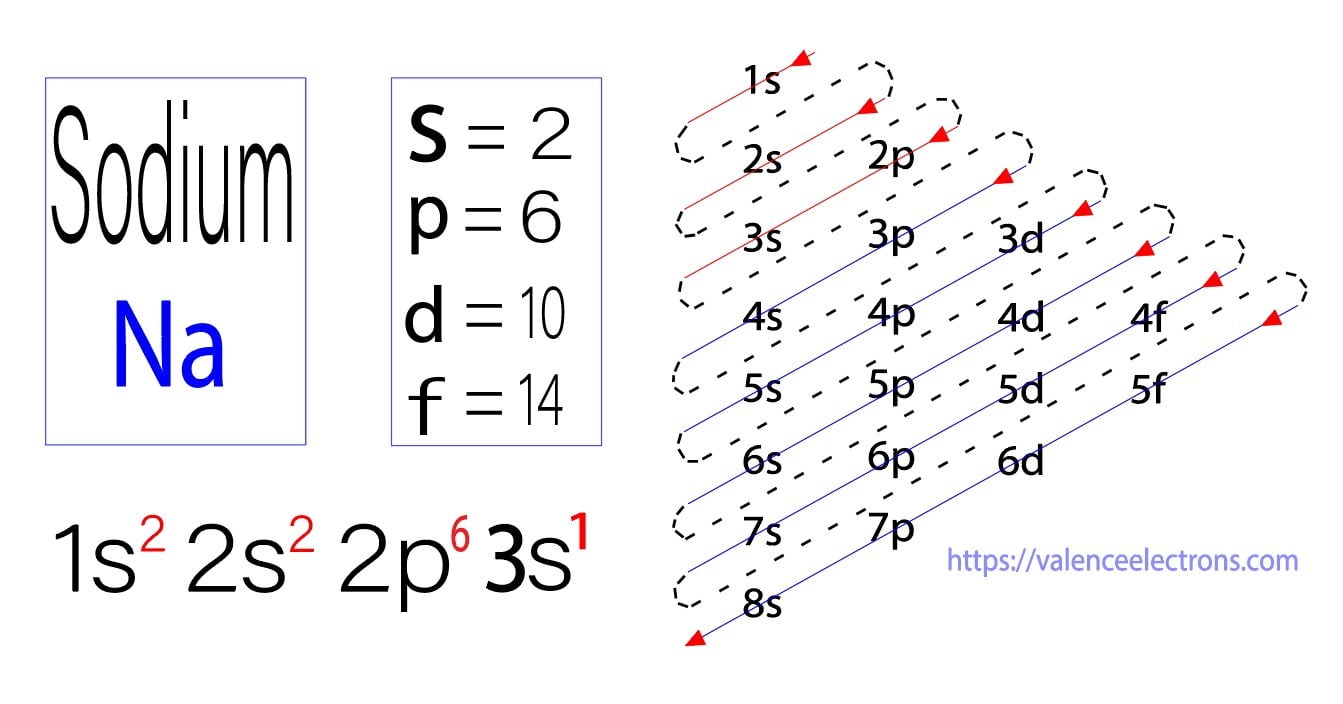
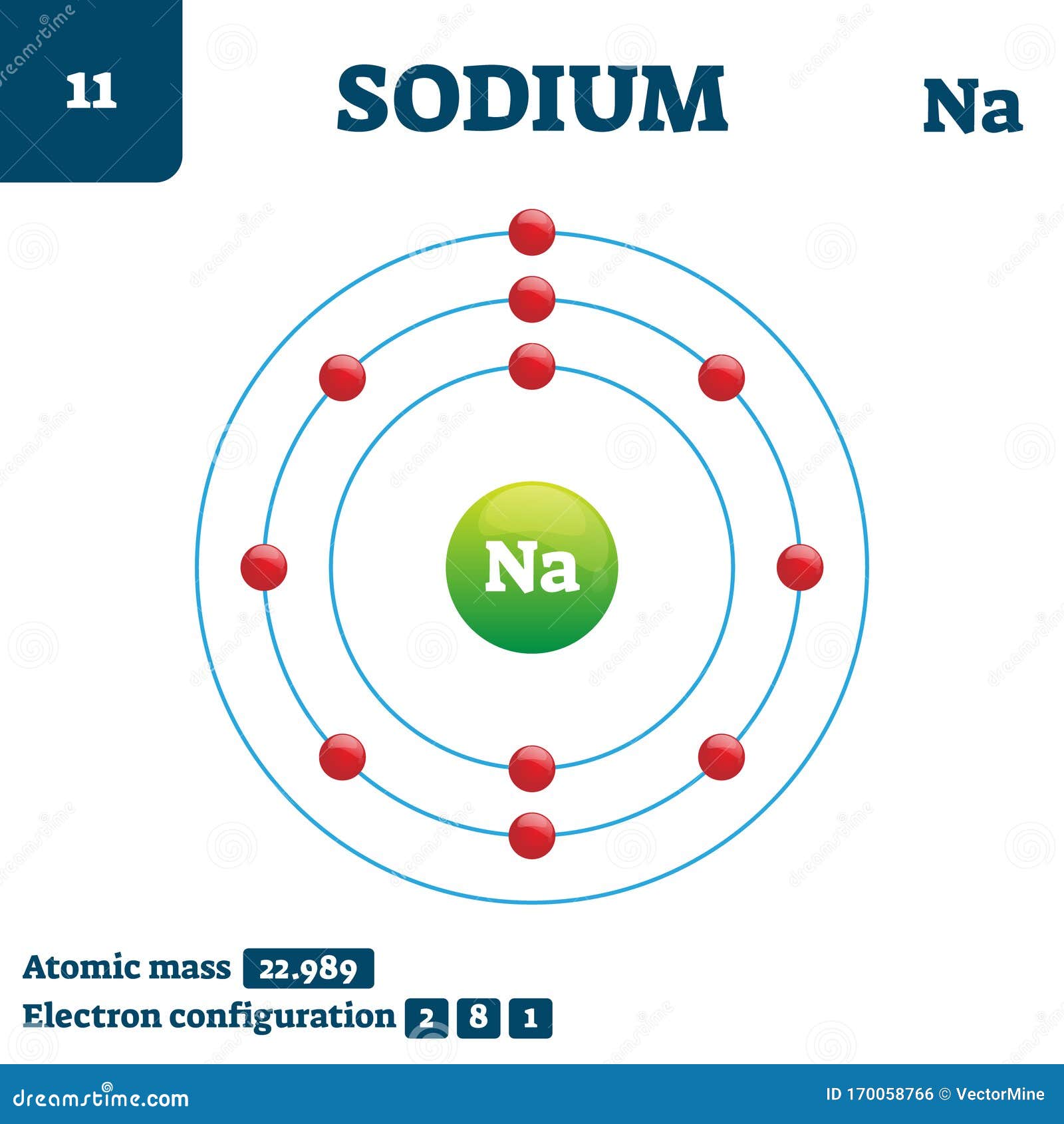
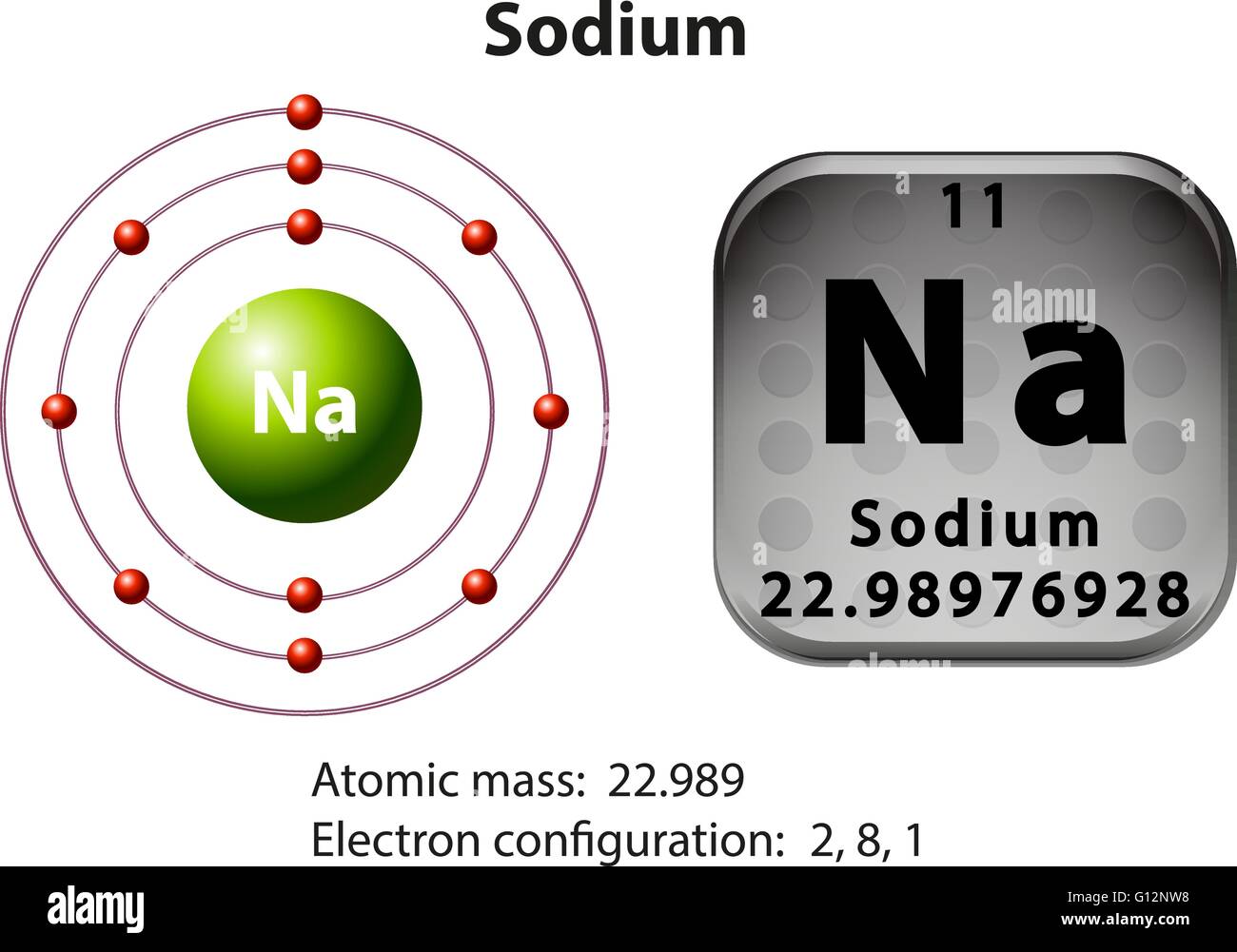
The electron configuration of a neutral sodium atom is 1s^2 2s^2 2p^6 3s^1. In this configuration we note that there is only one electron in the 3rd energy level. Atoms prefer to gain the stability of octet, by having eight electrons in the outer shell, the electrons of the s and p orbitals.

Atom Sodium Model Stock Illustration Download Image Now iStock
1 to 20: Predicting an electron arrangement The electron arrangement of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Sodium atoms.
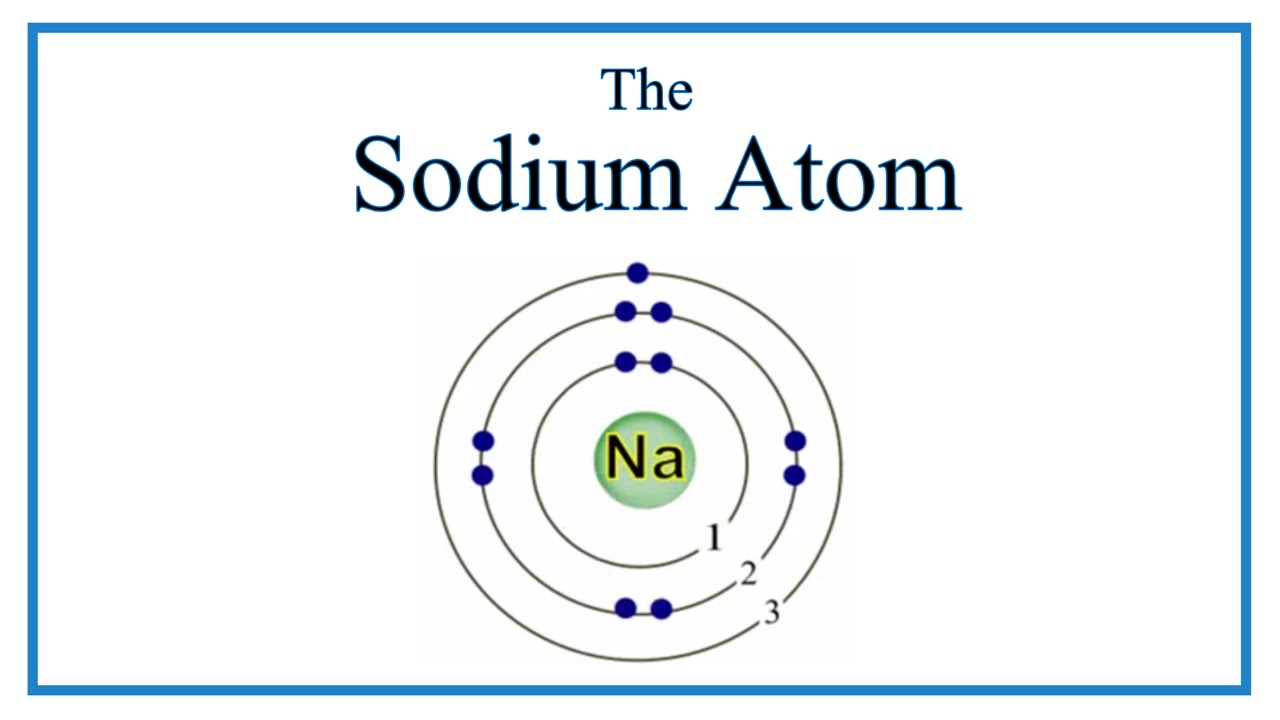
Atomic Structure of the Sodium Atom (Na) YouTube
The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. Orbital Energies and Atomic Structure.. Sodium cation loses one electron, so Na +: 1s 2 2s 2 2p 6 3s 1 = Na +: 1s 2 2s 2 2p 6. P: 1s 2 2s 2 2p 6 3s 2 3p 3.
Sodium Electron Configuration Photograph by Photo
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
Sodium Electron Configuration Electron Configuration Sodium What is
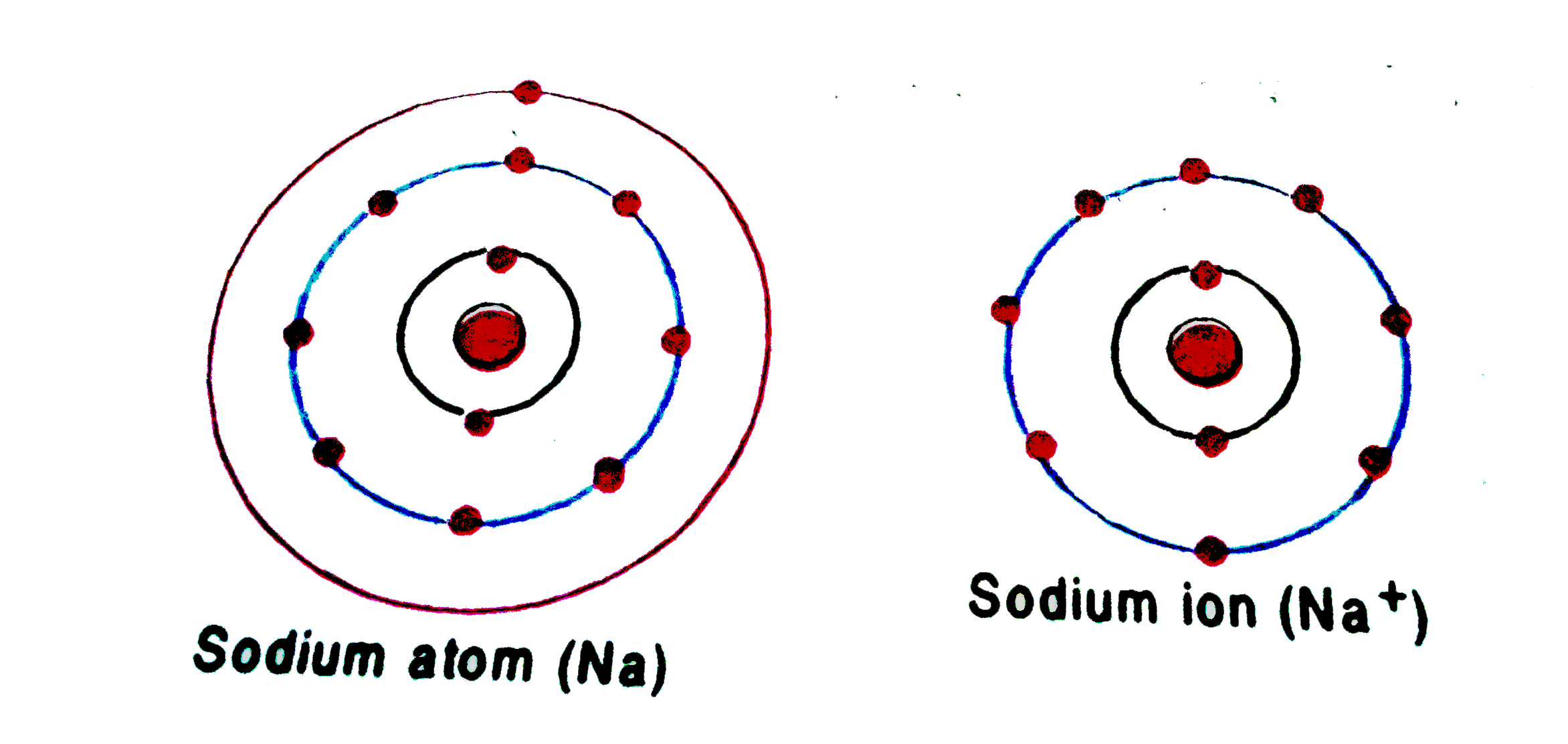
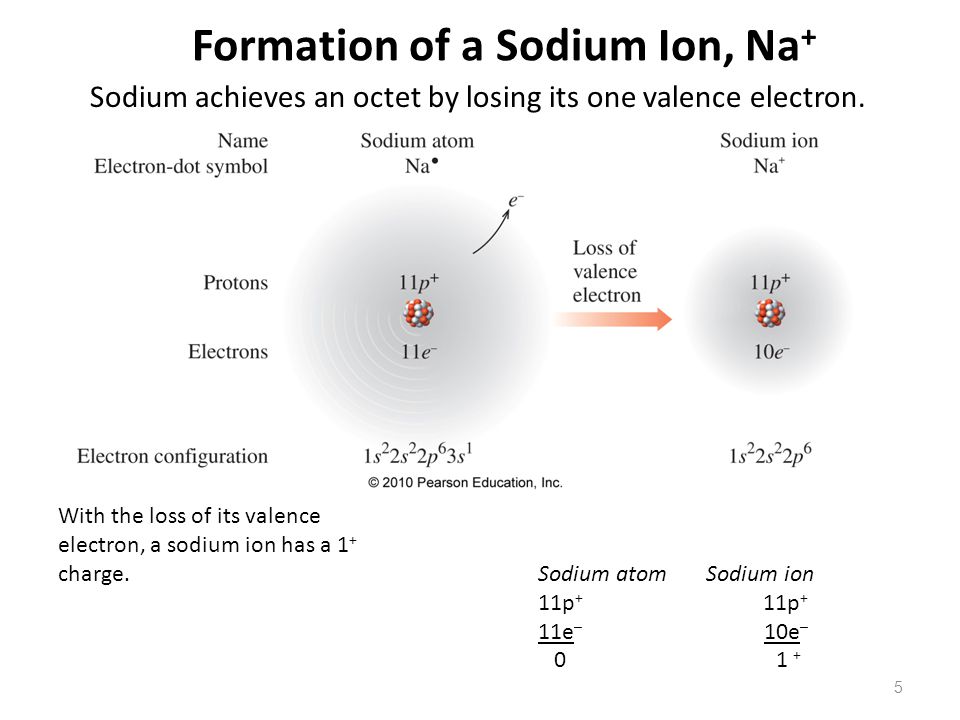
The atom that loses an electron becomes a positive ion. The atom that gains an electron becomes a negative ion. A positive and negative ion attract each other and form an ionic bond. Summary Students will look at animations and make drawings of the ionic bonding of sodium chloride (NaCl).
Electron Configuration for Sodium (Na, and Na+ ion)
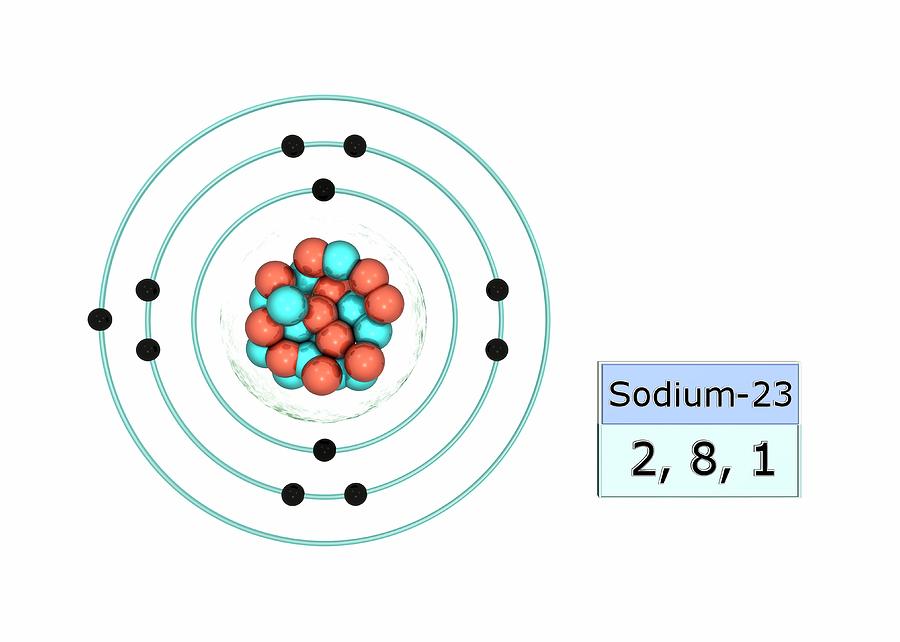
Stable Isotopes Electrons and Electron Configuration The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Sodium is 11.
Sodium Electron Configuration (Na) with Orbital Diagram
The electron arrangement of the sodium ion is now the same as that of the noble gas neon. Consider a similar process with magnesium and aluminum. In this case, the magnesium atom loses its two valence electrons in order to achieve the same arrangement as the noble gas neon and a charge of \(2+\). The aluminum atom loses its three valence.

Sodium Atom Science Notes and Projects
The electron arrangement of an element is related to its position on the periodic table. The electron arrangement of sodium (2.8.1) shows that sodium, Na: is in period 3 is in group 1.

Electron Configuration for Sodium (Na, and Na+ ion)
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid-liquid phase change occurs.. Sodium is essential to all living things, and humans have known this since prehistoric times. Our bodies contain about 100 grams, but we are constantly losing sodium in.
Sodium Electron Configuration (Na) with Orbital Diagram
We can show the electron arrangement as (2, 8, 2) representing the electrons in the n = 1 n = 1, n = 2 n = 2, and n = 3 n = 3 levels, respectively. Figure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3.

Diagram representation of the element sodium Vector Image
If we look at the arrangement of electrons in a sodium atom, we will find that two electrons fill the first shell, eight electrons fill the second shell, and there's one electron left to go in the third shell. But the question remains.

FileElectron shell 011 sodium.png Wikimedia Commons
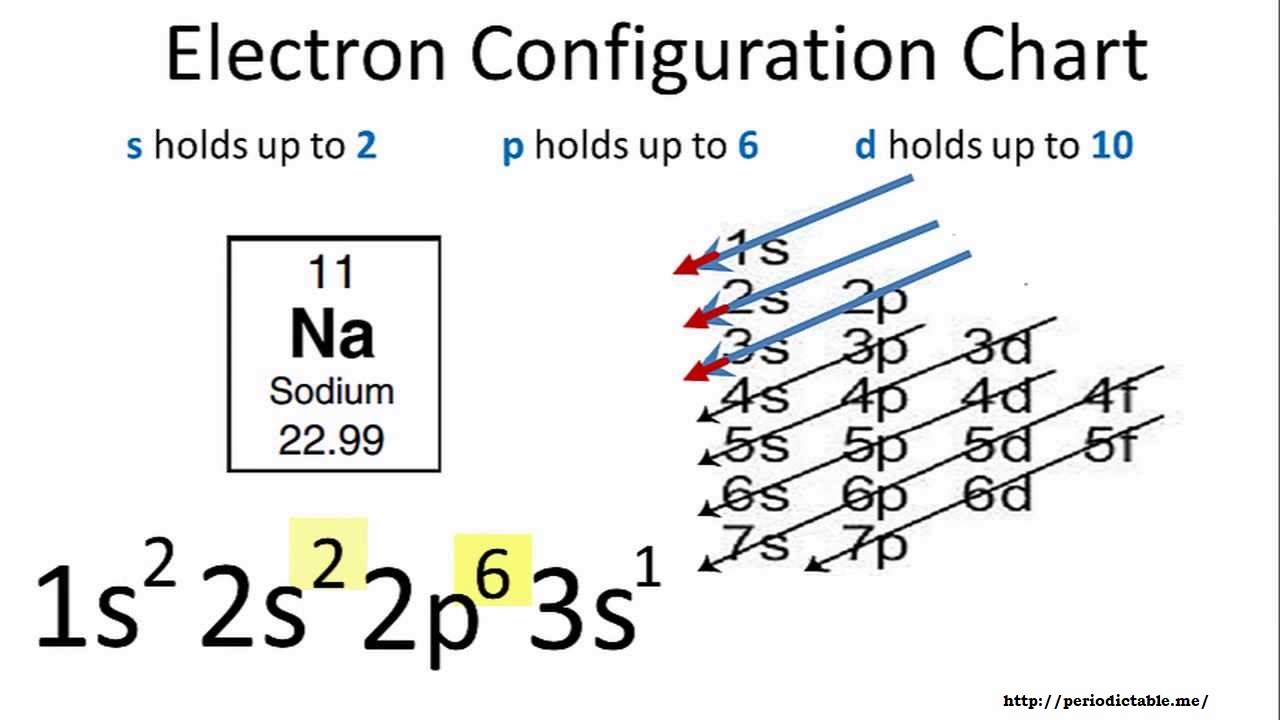
A step-by-step description of how to write the electron configuration for Sodium (Na). In order to write the Na electron configuration we first need to know.
Sodium Orbital Diagram
Sodium (Na) is the first element in the 3rd row (Period 3) in the periodic table. This means that the first shell and second shells of Na atom are filled to the maximum number of electrons. The first shell (1s) is filled with 2 electrons. The second shell (2s and 2p) has a total of 8 electrons. And, the third (last) shell has 1 electron.
Sodium atom polizworth
Because lithium's final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s 2 2s 1. The shell diagram for a lithium atom is shown below. The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1 s , while the outermost shell ( 2 s ) has 1 electron.
Symbol and electron diagram for Sodium illustration Stock Vector Image
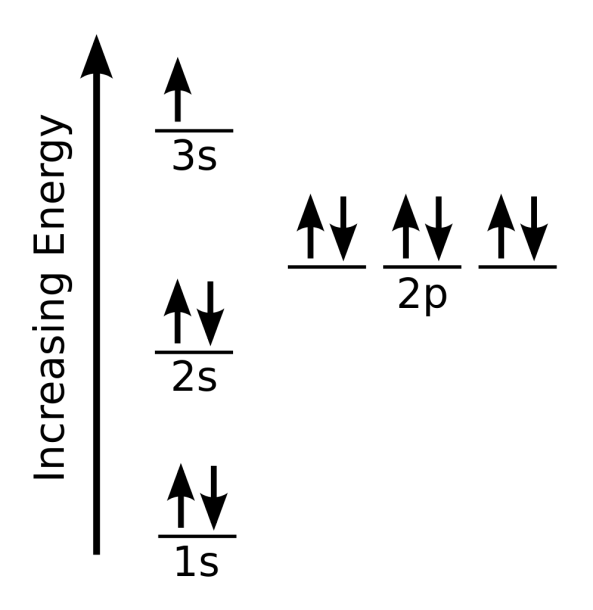
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\).. An orbital diagram is the more visual way.

Sodium electronic configuration How to Write Sodium electronic
March 23, 2023 by Jay. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.