
Power Point Water Cycle PDF
Triple point of water is a single point in P-T phase diagram of water where the three phases of water coexist. For practical applications people use the triple point cells. This video explains how to realize the triple point in this cell. To get to the triple point, temperature and Pressure should be set to specific values but what he shows in.

Water Point Hip Yick Industrial Company Limited
The Mathematics of Triple Point. The triple point of water is defined to take place at 273.16 K, where K is the SI unit Kelvin. Unlike Celsius and Fahrenheit scales, Kelvin is not measured using degrees; we merely say "Kelvin.". The triple point of water is important enough to merit its own line: T3 = 273.1K.

Freehold Rum Point Water Fron
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist, and is unique for every material. Water reaches its triple point at just above freezing (0.01° C) and at a pressure of 0.006 atm. Related reading: Thermodynamic equilibrium.
Water Drops Free Stock Photo Public Domain Pictures
Pure water has a triple point of 0. 01 ° C (273. 16 K, 32. 01 ° F) and pressure of 4. 58 mm (611. 2 Pa). Boiling point. The boiling point is defined as the point at which liquid starts boiling. At this point, matter exists in two states known as gaseous and liquid. The boiling point of water is 100 ° C. Difference between Boiling point and.

Water Drops Free Stock Photo Public Domain Pictures
ABSTRACT. This paper is a part of guidelines, prepared on behalf of the Consultative Committee for Thermometry, on the methods how to realize the International Temperature Scale of 1990. It discusses all major issues linked to the triple point of water when used as a fixed point for the realization of the kelvin. 1. Introduction.

Phase diagram with a triple point O of water analogy. Download
Triple point of water. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in equilibrium. It is a specific case of thermodynamic phase equilibrium. The term "triple point" was coined by James Thomson in 1873.

Flowing Water Free Stock Photo Public Domain Pictures
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist, and is unique for every material. Water reaches its triple point at just above freezing (0.1° C) and at a pressure of 0.006 atm. Notes: Currently only transportable to Thimann Lecture halls.
Our Properties Water Front Properties
In thermodynamics, the triple point of a substance is the unique combination of temperature and pressure at which solid phase, liquid phase, and gaseous phase can all coexist in thermodynamic equilibrium. By international agreement, the triple point of water has been assigned a value of 273.16 K (0.01 °C; 32.02 °F) and a partial vapor.

Vitapur Pointof Use TriTemperature Water Dispenser The Home Depot
Triple point of water is 273 K temperature and 0.46 cm of mercury pressure. The triple point of water, T 3 = 273.16 K, is the standard fixed-point temperature for the calibration of thermometers. This agreement also sets the size of the kelvin as 1/273.16 of the difference between the triple-point temperature of water and absolute zero.

Water Plunge Free Stock Photo Public Domain Pictures
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist.

B603CL Baritone Saxophone LIEN CHENG SAXOPHONE CO., LTD.
The triple point of water is fifteen-year-old Levi's entry into the Wolsey Hall Oxford Homeschooling Community Science Experiment competition.https://wolseyh.

TriValley Water Agencies Mandatory Water Usage Restrictions Remain in
A typical phase diagram.The solid green line applies to most substances; the dashed green line gives the anomalous behavior of water. In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. It is that temperature and pressure at which the sublimation, fusion.

Combining Proven Brands to Provide Our Customers with Reliable
TRIPLE-POINT CELL is a vacuum-tight flask containing water vapor, liquid water and an ice mantle that has formed around an inner test tube. To reach the triple point, first chill the cell.
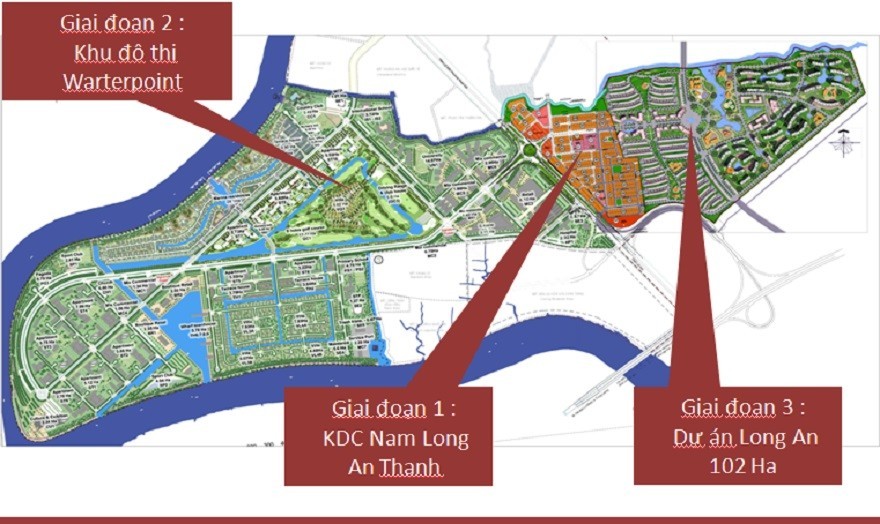
WaterPoint Bến Lức tận hưởng tiện ích đẳng cấp/htland.vn/
There are over 40 million people under a severe storm threat on Tuesday, according to the latest from the Storm Prediction Center. An enhanced risk for severe storms, or a level 3 of 5, is in.

The Water Droplets Free Stock Photo Public Domain Pictures
Hundreds of thousands of West Virginians were left without clean water after a chemical leak got into the Elk River on Jan. 9, 2014. Elaine Tanner told Eyewitness News she tried to get bottles of.

Water! The benefits of drinking enough (and how to do it!)
It is well known to scientists that the three common phases of water - ice, liquid and vapor - can exist stably together only at a particular temperature and pressure, called the triple point.