Scope of the hydroarylation of 2a with arenes. All yields are of
Our catalyst, MnBr (CO) (MeCN), avoids the formation of the off-cycle manganacycle- (CO) species responsible for low catalyst activity, allowing near room temperature hydroarylation of alkenes and alkynes with broad functional group tolerance including late stage functionalisation and diversification of bioactive molecules. PDF (8440K)

Síntesis de los complejos fac[MnBr(CO)₃(κ²P,Pdppm)] y cis,mer[MnBr
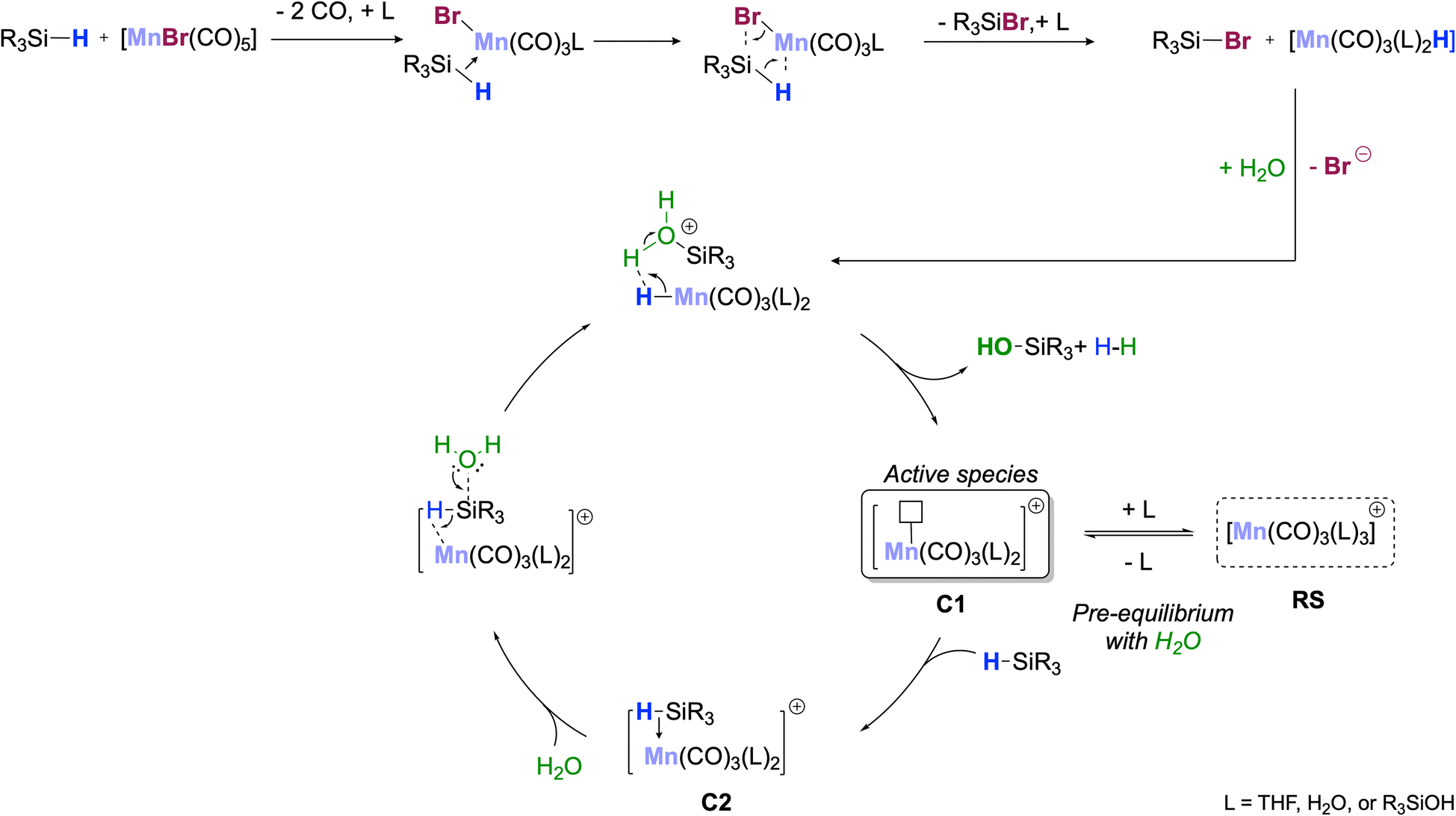
on computational and experimental studies, Wang and co-workers have proposed a mechanism where MnBr(CO)5 is a precatalyst forming o -cycle species I (Scheme 1b), which ff must dissociate CO and coordinate the alkyne coupling partner to enter the catalytic cycle as II.

Angew:Mn(I)/Ag(I)接力催化C(sp2)H/C(sp3)H偶联合成β(杂)芳基/烯基酮_CBG资讯
The pKa of HMn(CO)X5 H M n ( C O) X 5 in water is 7.1 7.1. This means that the Mn(CO)X5− M n ( C O) X 5 − complex exists, implying a −1 − 1 oxidation state for Mn M n. Again, I am not sure that a metal can adopt a negative oxidation state. As you can see they sure can get neg. ox. state, but because of such cases as this one, this doesn.
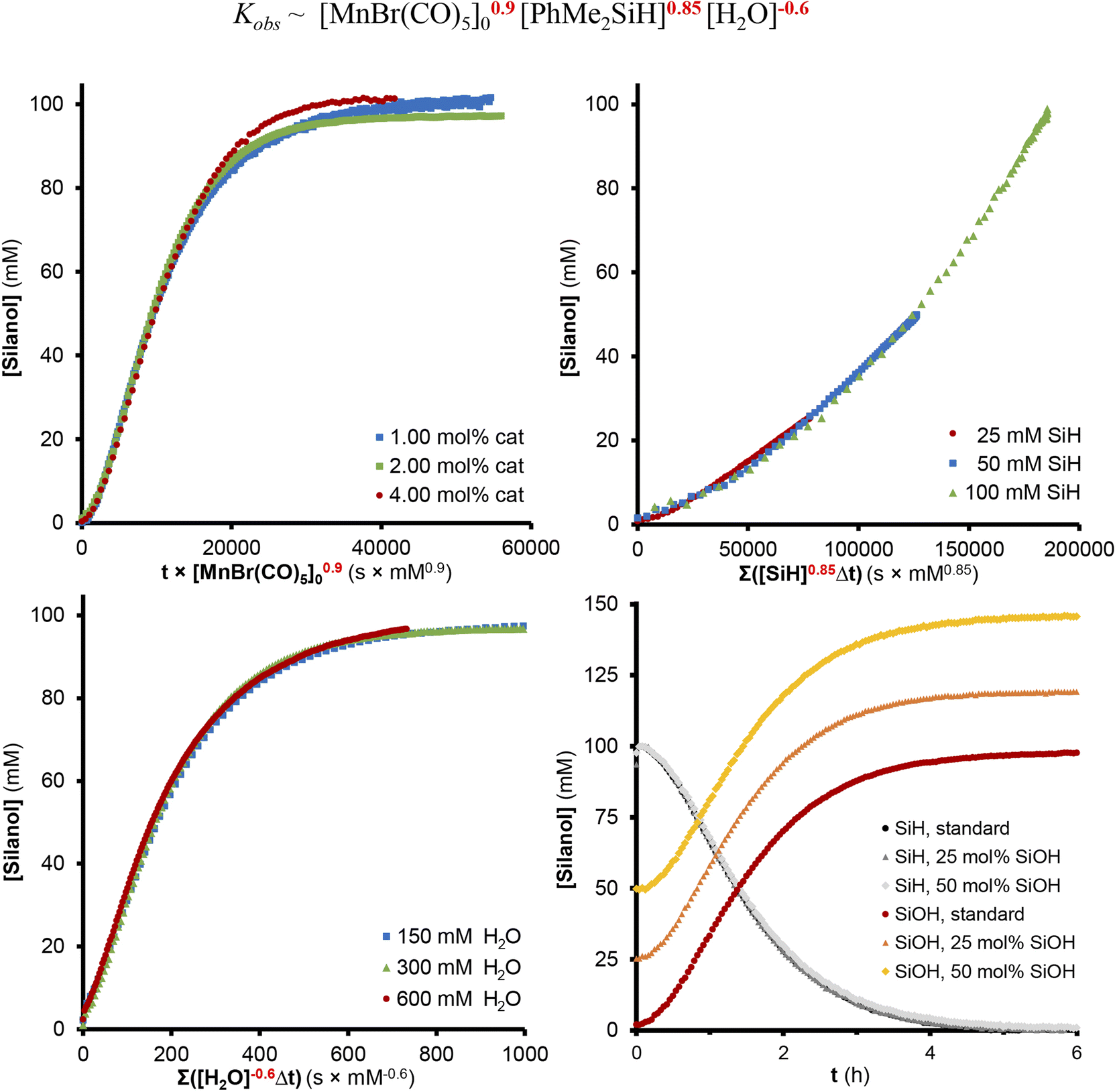
Selective oxidation of silanes into silanols with water using [MnBr(CO
Request PDF | MnBr(CO) 5 : a commercially available highly active catalyst for olefin hydrosilylation under ambient air and green conditions | The catalytic hydrosilylation of alkenes is a major.

13 C NMR spectrum of 4 (in DMSOd 6 ). Download Scientific Diagram
The reduction of carboxylic acids to the respective alcohols, in mild conditions, was achieved using [MnBr (CO) 5] as the catalyst and bench stable PhSiH 3 as the reducing agent.
Selective oxidation of silanes into silanols with water using [MnBr(CO
Here, we demonstrate that [MnBr(CO)5] is a highly active precatalyst for this reaction, operating under neutral conditions and avoiding the undesired formation of siloxanes. As a result, a broad substrate scope, including primary and secondary silanes, could be converted to the desired products.

Panel A yield−time profile performed on phenylacetic acid in
MnBr (CO)5: a commercially available highly active catalyst for olefin hydrosilylation under ambient air and green conditions - Green Chemistry (RSC Publishing) Issue 19, 2023 Previous Article Next Article From the journal: Green Chemistry
Figure S1 FTIR spectrum of [MnBr( 13 CO)n(CO)5n] 2 mM in THF
Manganese pentacarbonyl bromide is an organomanganese compound with the formula BrMn (CO) 5. It is a bright orange solid that is a precursor to other manganese complexes. The compound is prepared by treatment of dimanganese decacarbonyl with bromine: [1] Mn 2 (CO) 10 + Br 2 → 2 BrMn (CO) 5

(PDF) Selective oxidation of silanes into silanols with water using
Catalytic activities of Mn(I) complexes derived from expensive MnBr(CO) 5 salt have been explored in various dehydrogenative transformations. However, the reactivity and selectivity of inexpensive high spin Mn(II) complexes are uncommon. Herein, we have synthesized four new Mn(II) complexes and explored switchable alkenylation and alkylation of.
Unless otherwise specified, all reactions were carried out using 1 (0.2
Results and Discussion. Complexes 1-4 have been isolated from the reaction of [MnBr(CO) 5] with one equivalent of the appropriate α-diimine ligand (bpy, phen, dafo and pyzphen) in dichloromethane at room temperature.Yellow to orange crystals of these complexes are stable under ambient conditions for several months. Despite previous reports on spectroscopic properties of 1 and 2, no.

Figure S2 Detail of the carbonyl region of the FTIR spectrum of
Here, we demonstrate that [MnBr (CO) 5] is a highly active precatalyst for this reaction, operating under neutral conditions and avoiding the undesired formation of siloxanes. As a result, a broad substrate scope, including primary and secondary silanes, could be converted to the desired products.

a) 1 HNMR spectra and b) 13 CNMR spectra of copoly(BrPMAAmcoHEMA
Herein, we describe a novel catalytic hydroarylation that takes advantage of a Mn-complex capable of mediating these reactions at room temperature or near room temperature, overcoming one of the current bottlenecks in efficient Mn-catalysis ( Scheme 1d ). Go to: Results and discussion

Scheme S1 Scope of the reaction isolated yields are given below each
341622 Bromopentacarbonylmanganese (I) Write a review 98% Synonym (s): Bromopentacarbonylmanganese (I), Manganese pentacarbonyl bromide Linear Formula: BrMn (CO)5 CAS Number: 14516-54-2 Molecular Weight: 274.89 EC Number: 238-522-8 MDL number: MFCD00049806 PubChem Substance ID: 24860973 NACRES: NA.23
5S Circle Ribcon
The first manganese-catalyzed aromatic C-H alkenylation with terminal alkynes is described. The procedure features an operationally simple catalyst system containing commercially available MnBr(CO)(5) and dicyclohexylamine (Cy(2)NH). The reaction occurs readily in a highly chemo-, regio-, and stereo.

Substrate scope for the [3 + 2] annulations giving exoolefinic
5 was used as received, stored under argon in a glovebox or under air. Alkenes were stirred overnight on activated alumina, degassed by freeze-pump-thaw cycles, and stored under argon in a glovebox or used without any purification depending on the reaction conditions.
Scope of the hydroarylation of olefins with 1a. All yields are of
MnBr(CO)5: a commercially available highly active catalyst for olefin hydrosilylation under ambient air and green conditions @article{Vivien2023MnBrCO5AC, title={MnBr(CO)5: a commercially available highly active catalyst for olefin hydrosilylation under ambient air and green conditions}, author={Anthony Vivien and Laurent Veyre and Raphael.