What is Electricity?
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
OnlineLabels Clip Art Atom
Do you want to learn how to build an atom from scratch? Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. You can also play a fun game to check your understanding of atomic concepts. This simulation is part of the PhET project, a leading provider of free online STEM resources.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
35 Label The Parts Of The Atom In The Diagram Below Labels For Your Ideas
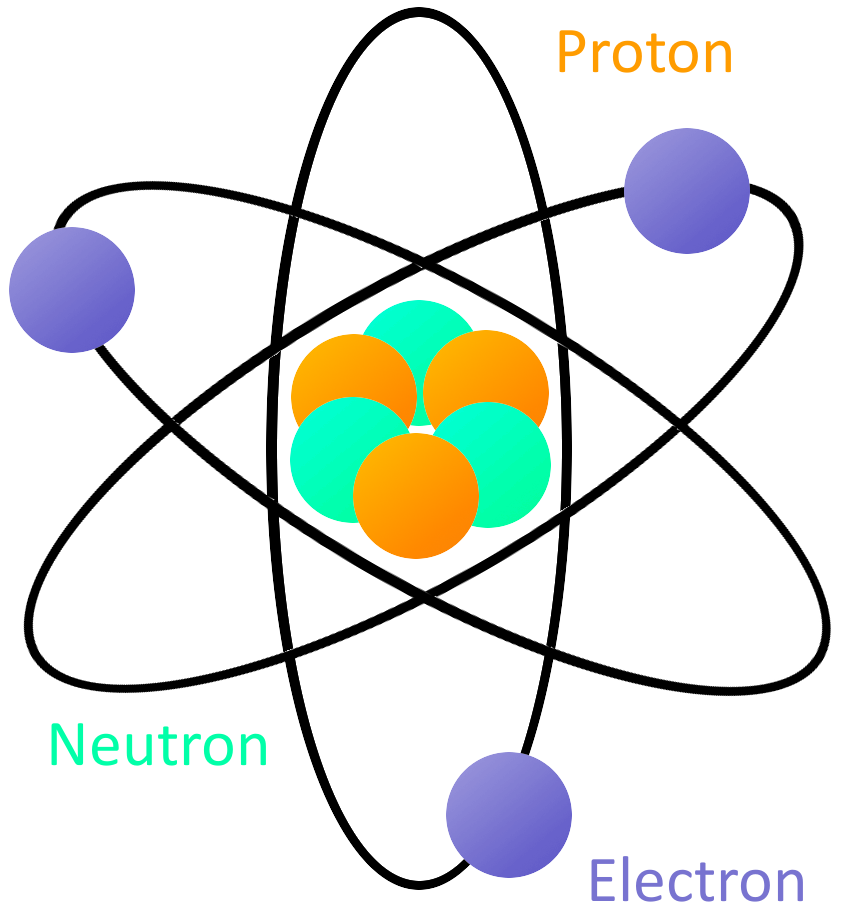
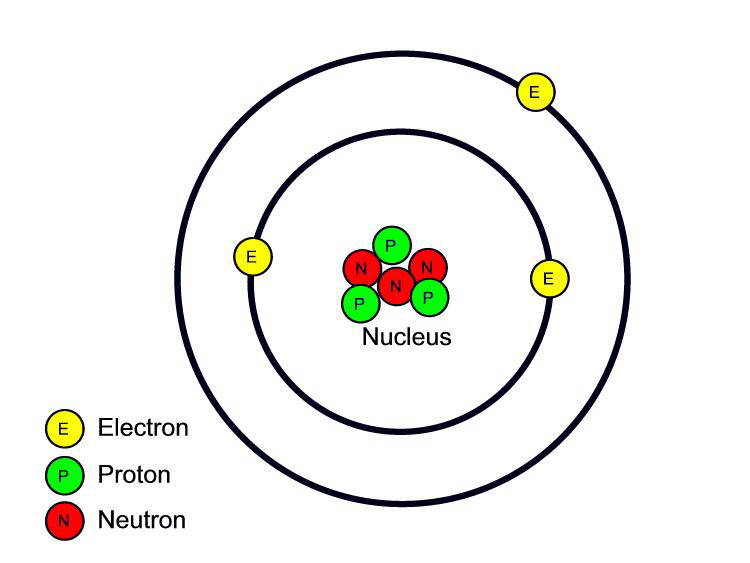
The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. J.J. Thomson Plum Pudding Model
Atom Free Stock Photo Public Domain Pictures
February 17, 2010 by Jerry Coffey Atom Diagram [/caption]The image on the left is a basic atom diagram. This one shows the protons, neutrons, and electrons of a carbon atom. Each is in a.

Atom Definition, Structure & Parts with Labeled Diagram
How does Niels Bohr's atomic model work? An overview of Niels Bohr's refinement of the Rutherford model. See all videos for this article Bohr model of the atom In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus. The orbits are labeled by an integer, the quantum number n.

The Nucleus of the Atom and Radioactivity
In the years after Dalton described his atomic model, multiple experiments were performed that proved that charged particles exist. In 1897 English physicist J.J. Thomson discovered a negatively charged particle, which he called the electron.The existence of the electron showed that the 2,000-year-old conception of the atom as a homogeneous particle was wrong and that in fact the atom has a.

عدد اتمی هیدروژن كنج كونج
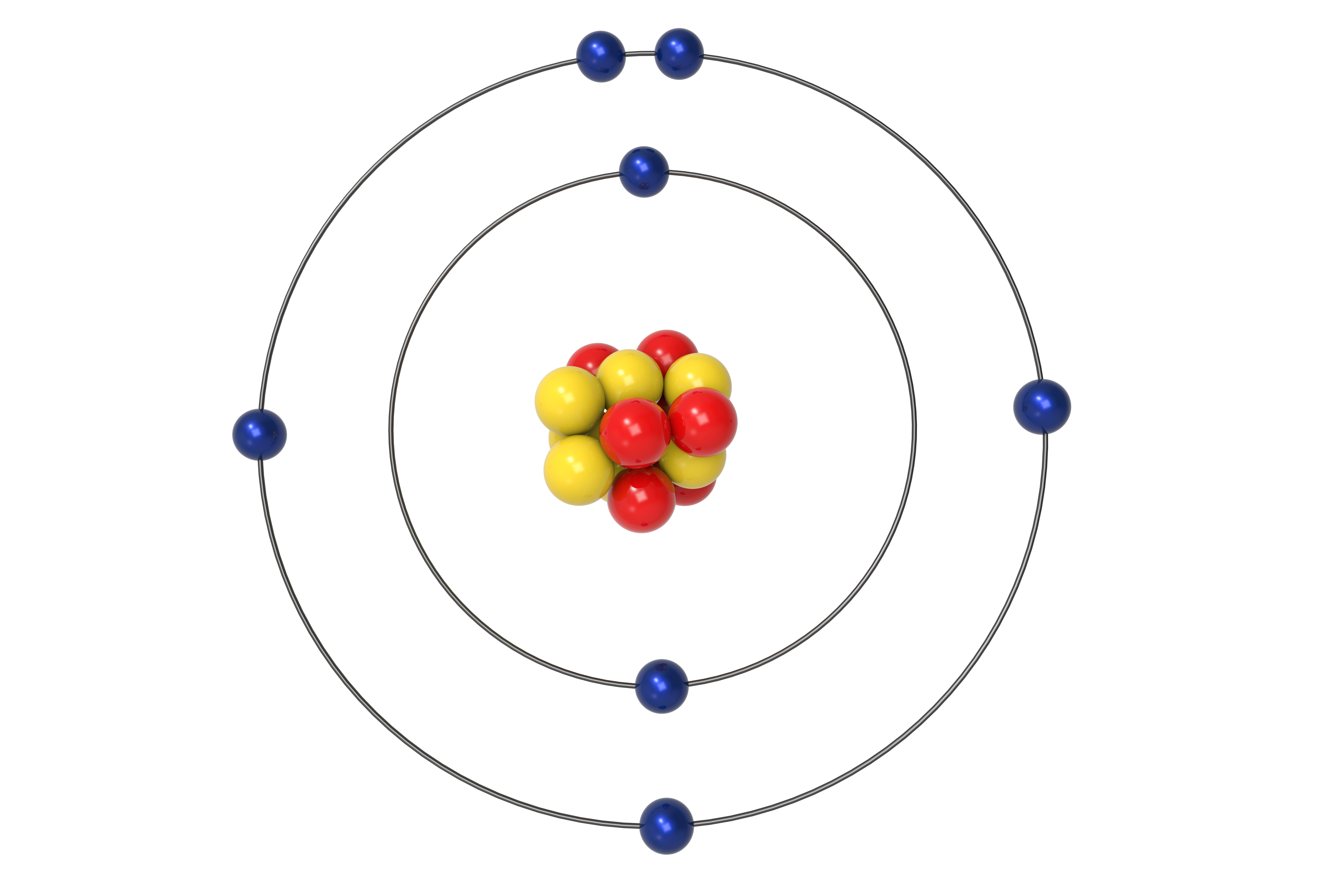
An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885-1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.
atom diagram to label
What is an atom? Are all atoms the same size? What does the mass of an atom consist of? How is the atomic number of an atom defined? atoms How atoms can be seen.

Atom American Welding Society Education Online
1 Answer Reyan Roberth · Stefan V. Jun 6, 2018 There are five basic atomic models which have contributed the structure of the atom itself. Explanation: They are: ⇒ John Dalton's atomic model: Dalton´s Billiard Ball (Solid Sphere) Model ⇒ J.J. Thomson's model: Plum Pudding model ⇒ Ernest Rutherford's model: Nuclear model

Modern Atomic Model Diagram PB Bohr Diagram Elsavadorla
The current theoretical model of the atom involves a dense nucleus surrounded by a probabilistic "cloud" of electrons. Atomic theory is the scientific theory that matter is composed of particles called atoms.The concept that matter is composed of discrete particles is an ancient idea, but gained scientific credence in the 18th and 19th centuries when scientists found it could explain the.
Chemistry Mysteries Atomic Structure & History of the Atom
In 1915, the Danish physicist Niels Bohr proposed a new model of the atom that involved electrons orbiting the nucleus. Stationary states or energy levels would be fixed distances from the nucleus (see below). In this model, these energy levels or shells would be represented by the letter "n."

Rutherford model Definition & Facts Britannica
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.
FileSchematicky atom.png Wikimedia Commons
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.
Atom Coloring Page Labeled Quantum Mechanical Model Of Atom , Free
5.10: Bohr Model of the Atom; 5.11: Energy Levels and Sublevels; 5.E: Models of the Atom (Exercises) 5: Models of the Atom is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Back to top; 4.11: Potential and Kinetic Energy; 5.1: Electron Configuration;
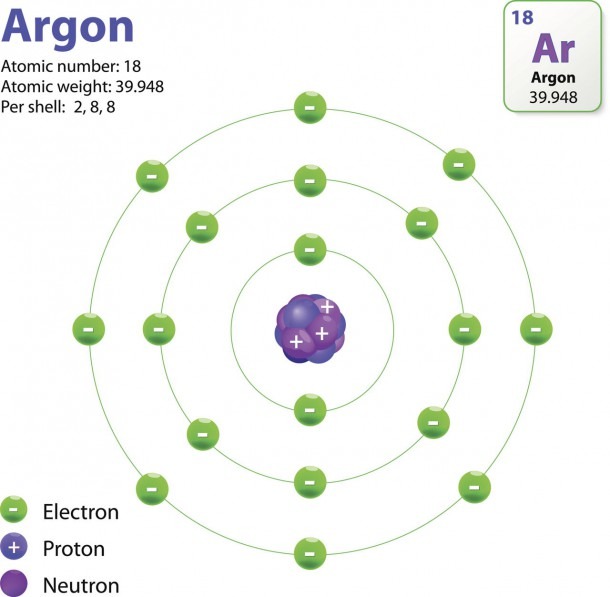
The Structure Of An Atom Explained With A Labeled Diagram Best
Our current model of the atom can be broken down into three constituents parts - protons, neutron, and electrons. Each of these parts has an associated charge, with protons carrying a positive.

Atom Free Stock Photo Public Domain Pictures
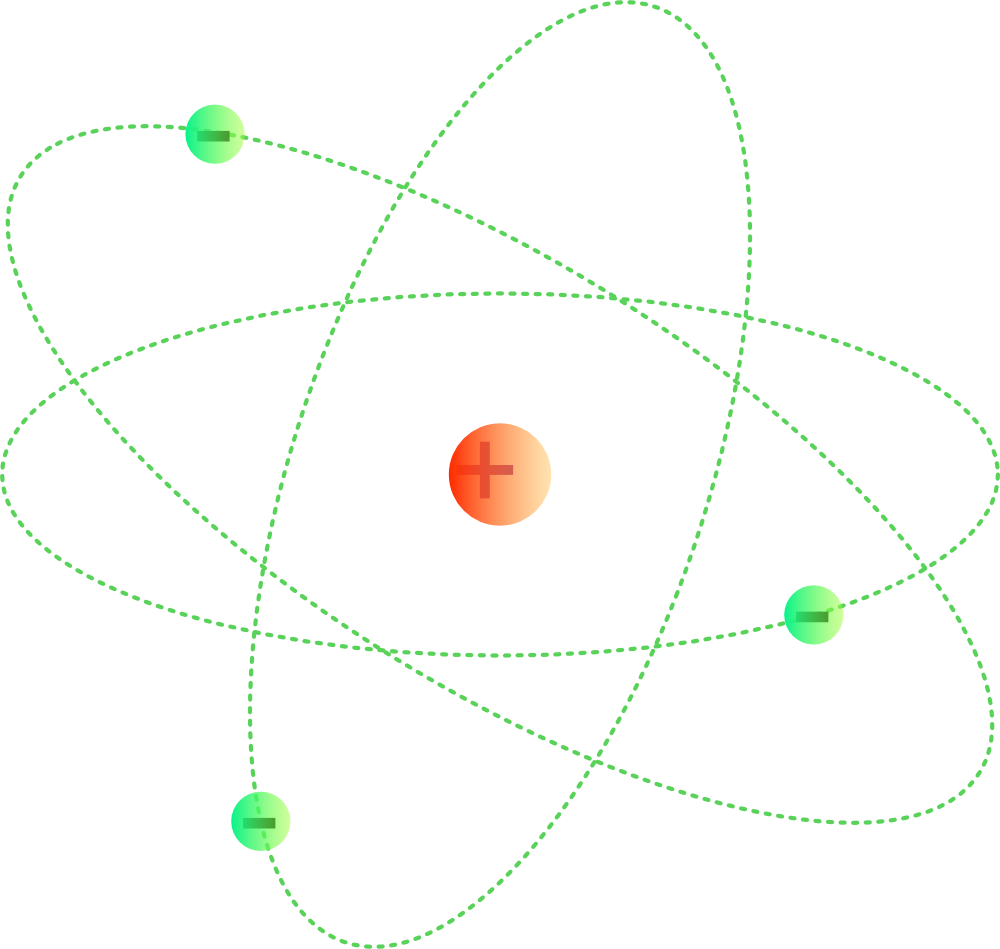
The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun. Rutherford gold-foil experiment