Best Overview Is CH3F Polar or Nonpolar Science Education and Tutorials Water is considered a polar solvent. Which substances should dissolve in water? methanol (CH 3 OH) sodium sulfate (Na 2 SO 4) octane (C 8 H 18), a non-polar organic compound; Solution. Because water is polar, substances that are polar or ionic will dissolve in it. Because of the OH group in methanol, we expect its molecules to be polar.
Is Ch3Ch2Oh Polar Or Nonpolar? Understanding The Nature Of This Learn to determine if CH3OH (Methanol) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structur.
SOLVED Draw the bondline formulas for the following compounds Ethanol $(\ce{CH3CH2OH})$ Propane $(\ce{CH3CH2CH3})$. When my teacher takes this question up, he said that propane is non polar, which means it only interacts via london forces, which means it has the lowest boiling points out of these three. But how is propane non polar though, when I asked, he said "I don't have an answer for this, life is.
is ch3nh2 polar hello everyone in this question they have given ethanol that is CH3CH2OH here they have asked that this molecule is polar or nonpolar so here the right answer is polar it is polar molecule polar molecule see in case of ethanol it belongs to alcohol group so now I'm going to write the structure of ethanol once again here that is CH3CH2 here we have OH here as we know that oxygen is a most.
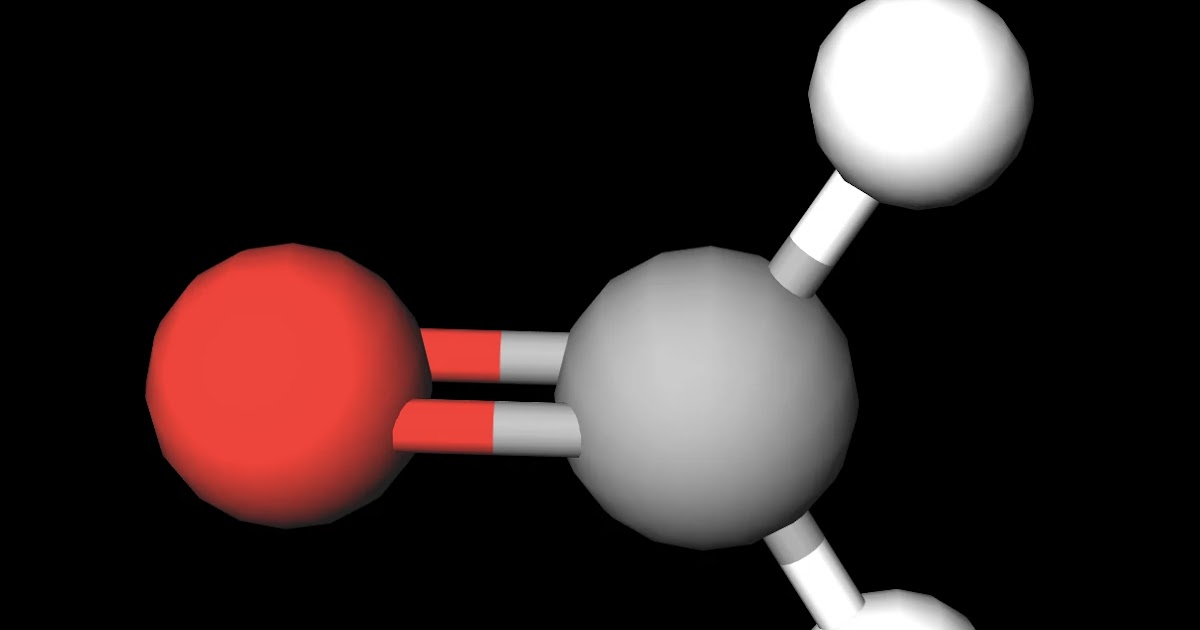
Is CH2O Polar or Nonpolar? Techiescientist Because CO is a polar molecule, it experiences dipole-dipole attractions. Because N 2 is nonpolar, its molecules cannot exhibit dipole-dipole attractions. The dipole-dipole attractions between CO molecules are comparably stronger than the dispersion forces between nonpolar N 2 molecules, so CO is expected to have the higher boiling point.
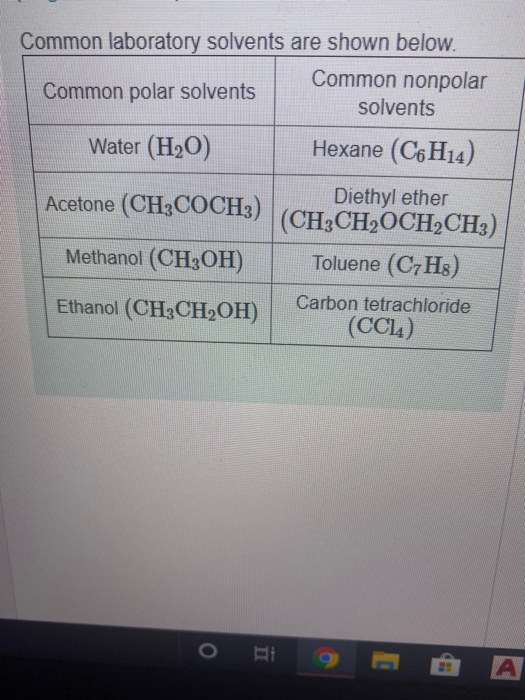
Solved Common laboratory solvents are shown below. Common Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer Ethanol molecule has an alkyl chain, and the O-H group is attached at the end of the.
Is O2 Polar Or Nonpolar? Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvent Polarity. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct.
Is CH3Cl Polar or Nonpolar? YouTube This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: d. (3 points) Is CH3CHO a polar or nonpolar molecule? If the molecule is polar, redraw the molecule showing the shape and include the molecular dipole.
VIDEO Is CH3CH2OH Polar or Nonpolar? Polarity of Ethanol H H ** ** ** H : C : C : O : H ** ** ** H H Q3. Give the electron pair and molecular geometries for each central atom. Q4. Give the bond polarity for each type of bond in the molecule. = There are two types of bonds in ethanol. The C-H bonds are nonpolar covalent. Q5. Is your molecule polar or nonpolar ? = Ethanol is both polar and nonpolar. Q6.
Lewis Structure For C2h5oh Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that.
POCl3 Lewis Structure (Phosphoryl Chloride) Lewis, Math, Molecules Exercise 2.12: Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar, hydrophobic 'solvent'. Decide on a classification for each of the vitamins shown below. Exercise 2.13: Both aniline and phenol are insoluble in pure water. Predict the solubility of these two compounds in 10% aqueous hydrochloric acid.
Is C2H5OH Polar or Nonpolar? (Ethanol) YouTube The boiling point of Methanol ( CH3OH) is 64.7 °C. The melting point of Methanol is -97.6 °C. The molecular weight of Methanol is 32.04 g/mol. It is a polar solvent and is also known as wood alcohol because it was once produced by the distillation of wood. The smell of this compound is on the sweeter side as compared to ethanol.
VIDEO science chemistry miscibility Fundamental Photographs The Art of Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that.
MakeTheBrainHappy Is CH2O Polar or Nonpolar? There are 4 steps to solve this one. Expert-verified Step 1 The compound CH A 3 CH A 2 CH A 2 OH, also known as propanol or 1-propanol, is a polar covalent molecule. To determine its. View the full answer Step 2 Unlock Step 3 Unlock Step 4 Unlock Answer Unlock Previous question Next question Not the question you're looking for?