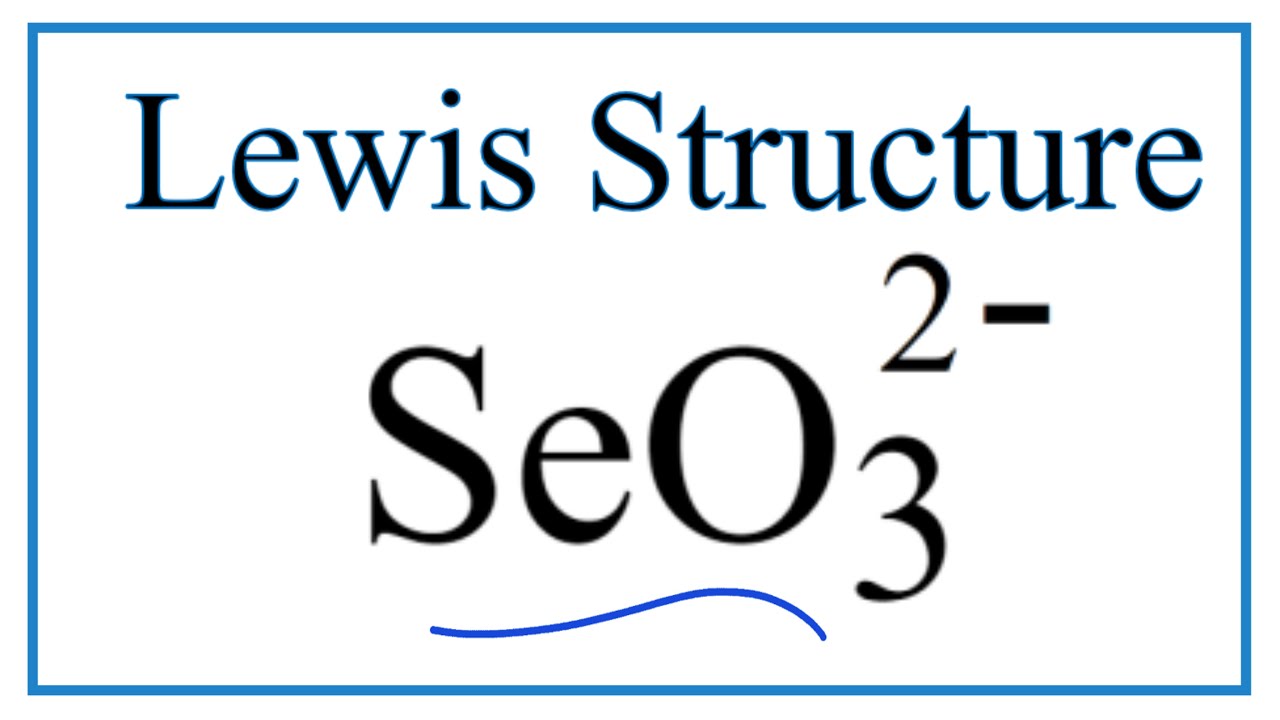
How to Draw the Lewis Dot Structure for SeO3 2 (Selenite ion) YouTube
But why? And how can you say that SeO2 is a polar molecule? Want to know the reason? Let's dive into it! SeO2 is a POLAR molecule because the Oxygen (O) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule.

Best Overview Is CH3F Polar or Nonpolar Science Education and Tutorials
1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom.

Determine whether CHCl3 is polar or nonpolar YouTube
Molecules with dipoles (polar molecules) contain polar bonds. lewis dot structure, molecular shape, atom electronegativity. is ClF2 polar or nonpolar. polar. is SeO3 polar or nonpolar. nonpolar. Hydrogen bonding. An unusually large dipole-dipole interaction, H "bonds" to a lone pair (on N, O, or F) only ones it can bond to.

Figure 2 from Polarnonpolar interconnected elastic networks with
Differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. Nonpolar covalent bonds have an equal distribution of electron density between the two nuclei. Polar covalent bonds have an unequal distribution of electron density with the more electronegative atom having greater electron.

Ch4 Polar Or Nonpolar / Solution Is The Ch4 A Polar Or Non Polar Chemistry
XeO3 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it. Let me explain this to you in 3 steps! Step #1: Draw the lewis structure Here is a skeleton of XeO3 lewis structure and it contains three Xe=O bonds.

Answered Complete the table below for these two… bartleby
Selenium trioxide is the inorganic compound with the formula Se O 3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se (VI) compounds. [4] Preparation Selenium trioxide is difficult to prepare because it is unstable with respect to the dioxide: 2 SeO 3 → 2 SeO 2 + O 2

CH2Cl2 polar or nonpolar Science education, Learning science, Science
Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances. Figure \(\PageIndex{14}\): (a) Molecules are always randomly distributed in the liquid state in the absence of an electric field. (b) When an electric field is applied, polar molecules like HF will align to the.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Covalent
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

Is CS2 polar or nonpolar? YouTube
SeO3 is a NONPOLAR molecule because all the three bonds (Se=O bonds) are identical and SeO3 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of SeO3 lewis structure and its 3D geometry. Why is SeO3 a Nonpolar molecule? (Explained in 3 Steps)

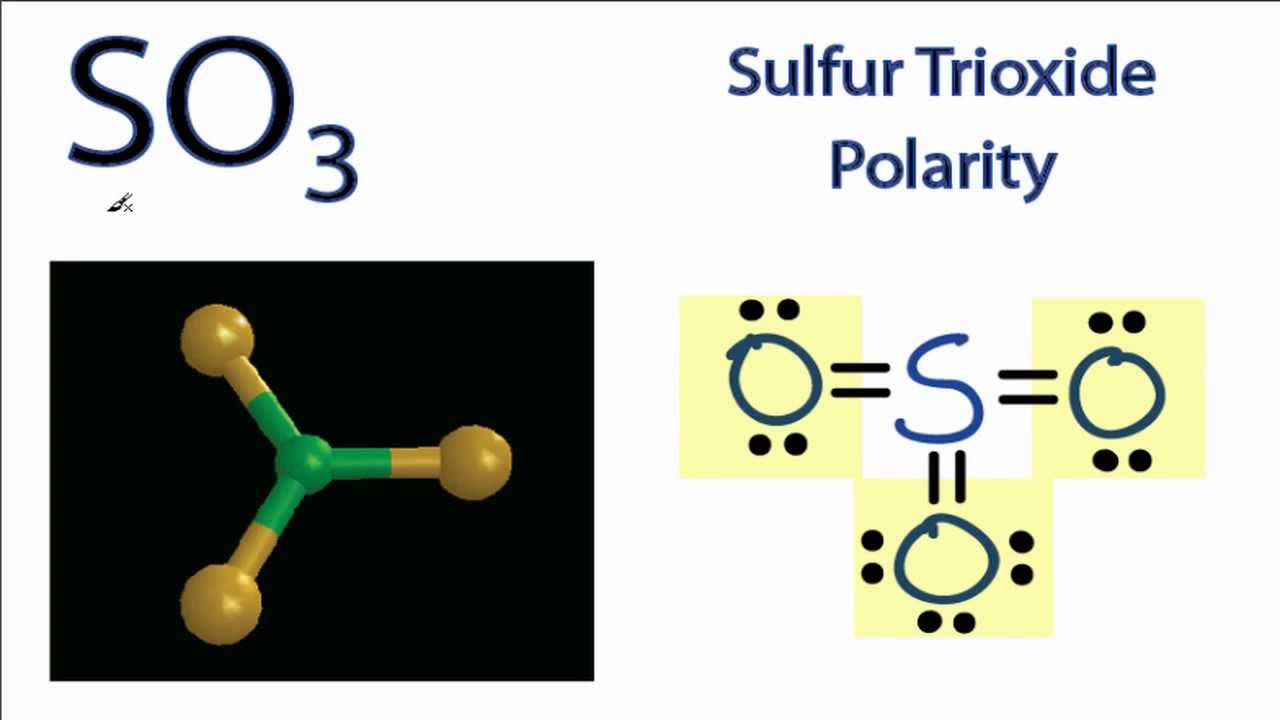
Is SO3 Polar or Nonpolar? Polarity of SO3
It also discusses if SeO3 is polar or nonpolar in addition to the bond angle, hybridization, and the molecular geometry. This video shows you how to draw the lewis dot structure of SeO3 also known as selenium trioxide. It also discusses if SeO3 is polar or nonpolar in addition to the bond angle, hybridization, and the molecular geometry..

Best Overview of Polar vs Nonpolar Molecules [No1] Science Education
If you look at the Lewis structure for SO3 it appears to be a symmetrical molecule. However, to determine if SO3 is polar we need to look at the molecular.
In So3 Polar Or Nonpolar?
H2SeO3 ⇌ SeO2 + H2O Let us study its lewis structure, geometry, and hybridization. Contents show Lewis Structure of Selenium Dioxide (SeO2) Also called the Lewis dot structure, it is a pictorial representation of the behavior and arrangement of valence electrons within an atom. It uses dots to represent valence electrons and lines to show bonds.
MakeTheBrainHappy Is CO Polar or Nonpolar?
Sulfur brings 6, and oxygen brings 3 each. That means; SO3 has 24 valence electrons. 6 + (3 x 6) = 24. Now have a look of Lewis Structure again; When we draw it, firstly we get the three structures at the top. Sulfur in the center and Oxygen around it is making a connection (each) to the central atom. There should be single bonds initially.

So2 Polar Or Nonpolar Asking List
As the bonds (S=O) are symmetrical and the SO3 molecule has a symmetrical geometry, their bond polarity gets canceled with each other. Because of this, there are no positive and negative poles of charges on the overall molecule of SO3. Hence, the SO3 molecule is a nonpolar molecule. I hope you have understood the reason behind the nonpolar.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
In summary, SeO3 is a nonpolar molecule due to its trigonal planar molecular geometry, which results in the cancellation of bond dipoles. Understanding the properties of SeO3, such as its nonpolarity, is crucial in various applications, including its use as a reagent in organic synthesis and as an oxidizing agent in chemical reactions.

Is SCN Polar or Nonpolar? Covalent bonding, Molecular geometry
Want to know the reason? Let's dive into it! SO3 2- is a POLAR ion because the Oxygen (O) atom is more electronegative and it also has lone pair, which results in an asymmetric shape of the ion. Because of this, the partial positive (ẟ+) and partial negative (ẟ-) charge appears on the ion.