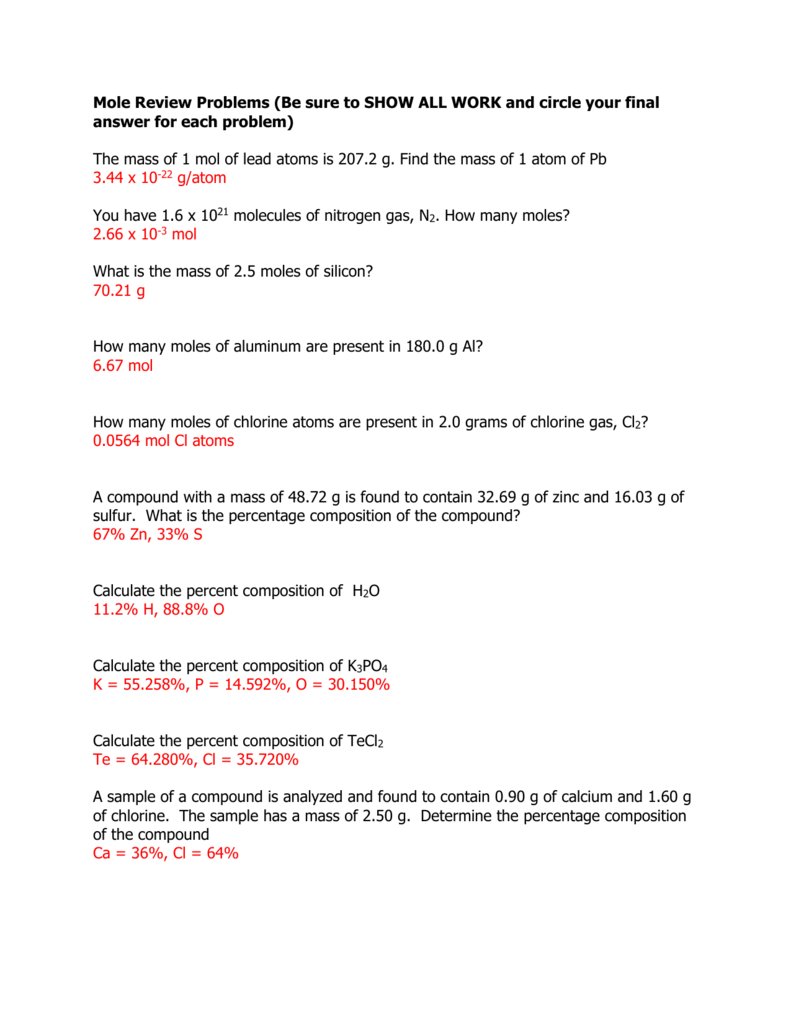
Worksheet Mole Problems Answers
1. N2 + 2O2 → N2O4 a. If 15.0g of N2O4 was produced, how many moles of O2 were required? = 0.326 mol O2 b. If 4.0x10-3 moles of oxygen reacted, how many grams of N2 were needed? = 5.6x10-2 g N2 2. K3PO4 + Al(NO3)3 → 3KNO3 + AlPO4 a. What is the mass of potassium nitrate that is produced when 2.04 moles of potassium phosphate react? = 619g KNO3 b.

12 Best Images of Chemistry Mole Practice Worksheet Mole Calculation
Title: Relative Mass and the Mole answer key Created Date: 20171005134609Z
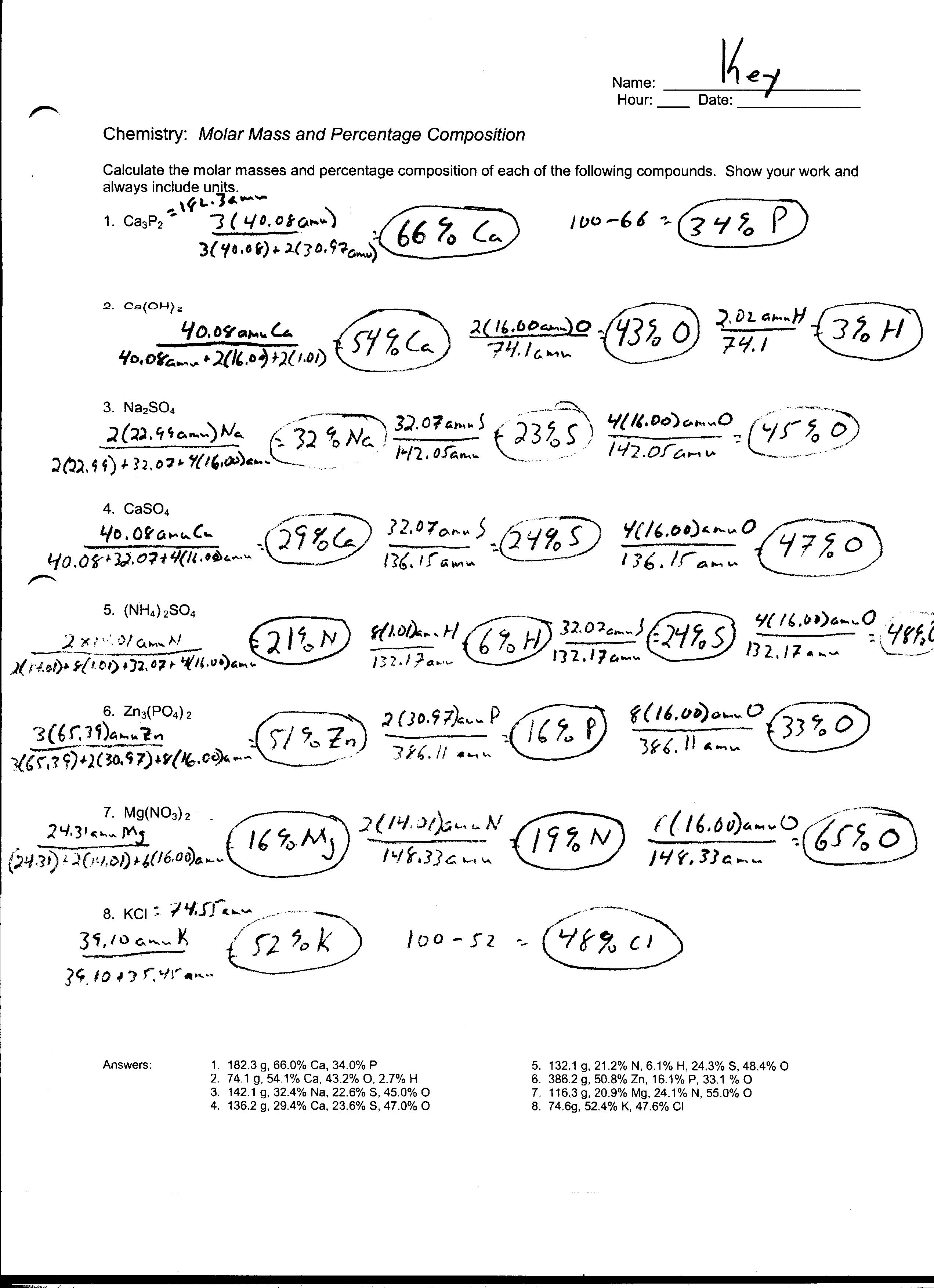
5 Chemistry If8766 Worksheet Answer Key /
Answer the following questions. 1. How many atoms are in 6.28 moles of aluminum? 2. How many atoms are in 90.43 moles of copper? 3. How many atoms in 7.64 moles of barium? 4. How many molecules in 3.72 moles of sulfur dioxide? 5. 78.54 g of nitrogen dioxide contain how many molecules? 6.

30++ Mole Calculation Worksheet
Answer each of the following questions using the equation provided. BE SURE TO BALANCE EACH EQUATION BEFORE SOLVING ANY PROBLEMS. SHOW ALL WORK. 1. ___NO 2 + ___O2 ___NO2 2 a. 2 moles of NO will react with ______ 1 mole(s) of O2 to produce ______ 2 mole(s) of NO2. b. ? moles NO = 3.6 moles O 2 moles NO 2 2 2 × 1 moles O 2 = 7.2 moles NO2 c.

The Mole Worksheets Answer Key
this is a chem worksheet that might have answers on it. Subject. Chemistry. 999+ Documents. Students shared 2783 documents in this course. Level Standard. School Woodhaven High School. Academic year: 2019/2020. Uploaded by:. Answer Key Stoichiometry: Mole-Mole Problems. 1. N2 + 3H 2 → 2NH 3.

Mole Conversions Worksheets Answer Key
Given the following equation, complete the questions below. 8Al + 3Fe3O4 → 4Al2O3 + 9Fe 8 A l + 3 F e 3 O 4 → 4 A l 2 O 3 + 9 F e. determine the number of moles of Fe F e produced from 2.0 moles of Al A l. determine the number of mol of Fe F e produced from 1.0 moles of Fe3O4 F e 3 O 4. determine the number of moles of Al2O3 A l 2 O 3.

Unit 7 Stoichiometry Mole Conversion Worksheet
answer The only new concept we will introduce in this unit is the idea of a mole. A mole is a quantity of matter that we use for conversion purposes. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles.

Mole Calculation Worksheet Answers Mole Calculation Worksheet
The production of ammonia (NH 3) from nitrogen and hydrogen gases is an important industrial reaction called the Haber process, after German chemist Fritz Haber. N 2(g) + 3H 2(g) → 2NH 3(g) The balanced equation can be analyzed in several ways, as shown in the figure below. Figure 12.2.3: This representation of the production of ammonia from.

30++ Mole Ratio Worksheet Answer Key Worksheets Decoomo
NewPathWorksheets offers chemistry study guides, worksheets & answer keys for high schoolers to understand the topic of the Mole. Convenient & printable Mole guides.

14 Electrostatics Worksheet A Concepts & Calculations Answer Key
Moles Molecules and Grams Worksheet How many molecules are there in 24 grams of FeF3? How many molecules are there in 450 grams of Na2S04? 24 How many grams are there in 2.3 x 10 atoms of silver? 23 How many grams are there in 7.4 x 10 molecules of AgN03? 23 How many grams are there in 7.5 x 10 molecules of H2S04?

18 Moles Worksheet With Answers /
Solve of the following: 1) How many moles are in 15 grams of lithium? 2) How many grams are in 2.4 moles of sulfur? 3) How many moles are in 22 grams of argon? 4) How many grams are in 88.1 moles of magnesium? 5) How many moles are in 2.3 grams of phosphorus? _molar mass (g) 1 mole OR __6.02 x 1023_ 1 mole Page 1 of 2

14 Mole Conversion Worksheet /
Answer 0.00119 mol C 4 H 8 O 2 which is 0.105 g C 4 H 8 O 2 7.1.2: Practice Mole Calculations is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.

Mole Worksheet 1 Answer Key Printable Word Searches
Mole to Mole Problems KEY Worksheet: Mole/Mole Problems Name Answer each of the following questions using the equation provided. BE SURE TO BALANCE EACH EQUATION BEFORE SOLVING ANY PROBLEMS. SHOW ALL WORK. NO + 02 N02 1. How many moles of N - will be produced if 3.6moles NO react? 2.

MOLE WORKSHEET
Description. Answer Key to Mole Conversion 2.0. 6 Questions. All answers included & all of the work is shown also. .docx file. Thechemteacher.weebly.com. The Chemistry Teacher on YouTube.

Mole Particle Conversions Worksheet Answers
For CO 2 (m.w. = 44.01 u), mole CO 2 = 44.01 g CO 2 = 6.022 x 10 23 CO 2 molecules. Because each CO 2 molecule is composed of one carbon atom and two oxygen atoms, we could say that a mole of CO 2 contains one mole of carbon atoms and two moles of oxygen atoms. In general, it is useful to think of a mole as just an Avogadro's number of things.

18 Best Images of Mole Conversion Problems Worksheet Answers Mole Ratio
a. 2.48 g of HBr. b. 4.77 g of CS 2. c. 1.89 g of NaOH. d. 1.46 g of SrC 2 O 4. 16. Decide whether each statement is true or false and explain your reasoning. There are more molecules in 0.5 mol of Cl 2 than in 0.5 mol of H 2. One mole of H 2 has 6.022 × 10 23 hydrogen atoms. The molecular mass of H 2 O is 18.0 amu.